Abstract
Background
Models of Alzheimer disease propose a sequence of amyloid-β (Aβ) accumulation, hypometabolism, and structural declines that precede the onset of clinical dementia. These pathological features evolve both temporally and spatially in the brain. This study aimed to characterize where in the brain and when in the course of the disease neuroimaging biomarkers become abnormal.
Methods
We analyzed data from mutation non-carriers, asymptomatic carriers, and symptomatic carriers collected between January 1st 2009 and December 31st 2015 from families carrying PSEN1, PSEN2, or APP mutations enrolled in the Dominantly Inherited Alzheimer’s Network. We analyzed [11C]Pittsburgh Compound B positron emission tomography (PiB PET), [18F]Fluorodeoxyglucose (FDG PET), and structural magnetic resonance imaging (MRI) data using regions of interest to assess change throughout the brain. We estimated rates of biomarker change as a function of estimated years from symptom onset at baseline using linear mixed-effects models and determined the earliest point at which biomarker trajectories differed between mutation carriers and non-carriers.
Findings
PiB PET was available for 346 individuals, with 162 having longitudinal imaging; FDG PET was available for 352 (175 longitudinal); and MRI data was available for 377 (201 longitudinal). We found a sequence to pathological changes, with rates of Aβ deposition in mutation carriers being significantly different from non-carriers first (on average across regions that showed a significant difference at −18·9 (sd 3·3) years before expected onset), followed by hypometabolism (−14·1 years, sd 5·1) and lastly structural declines (−4·7 years, sd 4·2). This biomarker ordering was preserved in most, but not all, regions. The temporal emergence within a biomarker varied across the brain, with the precuneus being the first cortical region in each modality to show divergence between groups (−22·2 years before expected onset for Aβ accumulation, −18·8 years for hypometabolism, and −13·0 years for cortical thinning).
Interpretation
Mutation carriers had elevations in Aβ deposition, reduced glucose metabolism, and cortical thinning which preceded the expected onset of dementia. Accrual of these pathologies varied throughout the brain, suggesting differential regional and temporal vulnerabilities to Aβ, metabolic decline, and structural atrophy, which should be taken into account when using biomarkers in a clinical setting as well as designing and evaluating clinical trials.
Introduction
Alzheimer disease (AD) presents as a progressive loss of cognitive function, leading to severe impairment and loss of independence. AD’s long preclinical phase has bolstered efforts to identify in vivo biomarkers to aid disease diagnosis and prognosis1. Models of AD pathophysiology theorize a temporal sequence where disruptions in amyloid-β (Aβ) production and/or clearance initiate a biological cascade that leads to Aβ plaque formation that spreads throughout the cortex followed by tauopathy, neuronal dysfunction and death, and ultimately dementia2,3.
Positron emission tomography (PET) and magnetic resonance imaging (MRI) can assess both the amount and location of Aβ plaques, tauopathy (neurofibrillary tangles, neuritic plaques, and neuropil), altered glucose metabolism, and structural decline. The temporal sequence of these biomarkers provides information about the pathogenesis of AD. Determining the ordering of changes in sporadic AD is problematic, as it is difficult to predict an individual’s relative position in the disease. Autosomal dominant AD (ADAD) is well suited to study biomarker trajectories due to the virtually complete penetrance of the mutations and consistency of symptom onset within families4,5. The conserved onset age within families and mutation types allows individuals to be staged relative to their expected onset of symptoms.
ADAD work has revealed a temporal ordering of biomarkers consistent with theoretical models,6–8 and indications that pathology progressively appears in new regions of the brain as the disease worsens7. This has primarily relied on cross-sectional analyses, with limited analyses of modest longitudinal cohorts7,9–16. Longitudinal analyses can provide a better estimate of the true pathological trajectories.17,18 This is critical as interventional trials such as the Dominantly Inherited Alzheimer Network (DIAN) trials unit,19, the Alzheimer’s Prevention Initiative (API),20 and the Anti-Amyloid Treatment in Asymptomatic Alzheimer’s Study (A4)21 will all evaluate alterations in longitudinal biomarker trajectories.
The DIAN observational study (DIAN)4 has established a large cohort of ADAD families with longitudinal Aβ, metabolic, and structural neuroimaging assessments. Our current work compares rates of biomarker change in a large population of mutation carriers (MC) and non-carriers (NC) throughout the entire brain. In this way we can visualize when pathology biomarkers first emerge and how they spread throughout the course of the disease.
Methods
Participants
Individuals from families known to have mutations in the presenilin 1 (PSEN1), presenilin 2 (PSEN2), and amyloid precursor protein (APP) genes were recruited from 14 performance sites participating in the DIAN observational study (http://www.dian-info.org). Participants were recruited from DIAN sites in the United States, Great Britain, Germany, and Australia between January 1st 2009 and December 31st 2015. All participants with genetic, clinical, and neuroimaging data that passed quality control from the tenth semiannual data freeze were included in the analyses. The institutional review board at Washington University in St. Louis provided supervisory review and human studies approval. Participants or their caregivers provided written informed consent in accordance with their local institutional review board. Clinical and imaging visits in DIAN are performed every three years for asymptomatic individuals until they are within three years of their parental age of dementia onset. Assessments become annual once an individual is within three years of parental age at onset or if an individual becomes symptomatic. Analyses excluded families with the Dutch and Flemish Mutation, as these APP mutations often present with predominant cerebral amyloid angiopathy and diffuse Aβ plaques (see supplemental material). The analyses included 346 individuals with Aβ PET data, 352 with PET metabolism data, and 377 with MRI.
Clinical Assessment
Dementia status was assessed using the Clinical Dementia Rating (CDR)22. For each visit a participant’s estimated years from expected symptom onset (EYO) was calculated based upon the participant’s current age relative to either the family mutation specific expected age at dementia onset5 or parental age at first progressive cognitive decline if mutation age at onset was unknown. A “mutation specific” expected age of dementia onset is calculated by averaging the age of onset reported in the literature across individuals with the same specific mutation5. EYO is established identically for both carriers and non-carriers. The presence or absence of an ADAD mutation was determined using PCR-based amplification of the appropriate exon followed by Sanger sequencing6. Clinical evaluators were blind to participant mutation status.
MRI
MRI was performed using the Alzheimer’s Disease Neuroimaging Initiative (ADNI) protocol23. Sites used a 3T scanner and were required to pass regular quality control assessments. T1-weighted images (1·1 × 1·1 × 1·2-mm voxels) were acquired for all subjects. The ADNI Imaging Core screened images for protocol compliance and artifacts. Volumetric segmentation and cortical surface reconstruction was performed using FreeSurfer 5·324,25 which automatically defines subcortical and cortical regions of interest (ROIs). Segmentations were inspected by members of the DIAN Imaging Core and edited as needed. Subcortical volumes were corrected for intracranial volume using a regression approach. Cortical thickness and volume measures were averaged across hemispheres. The cortical and subcortical labels identified on the MRI were utilized for the regional processing of all PET data. For all analyses we examined 34 cortical ROIs and 7 subcortical ROIs. A full list of regions is available in supplemental material.
PET
Aβ imaging was performed using a bolus injection of [11C]Pittsburgh Compound B (PiB). Acquisition consisted of a 70-minute scan starting at injection or a 30-minute scan beginning 40 minutes post-injection. Data in the common 40–70 minute time frame was converted to regional standardized uptake value ratios (SUVRs) relative to the cerebellar grey matter using FreeSurfer derived regions of interest26 (PET Unified Pipeline, https://github.com/ysu001/PUP). Metabolic imaging was performed with [18F]Fluorodeoxyglucose (FDG) with a 30-minute dynamic acquisition beginning 30 minutes after injection. Data from the last 20 minutes of each FDG scan were converted to SUVRs relative to cerebellar grey. Both types of PET data were partial volume corrected using a regional spread function technique27,28.
As there were no a priori laterality predictions, data were averaged across hemispheres before being entered into statistical analyses. Differences in spatial resolution across PET scanners were accounted for by applying scanner specific spatial filters to achieve a common resolution (8 mm)29. The ADNI PET Core verified that PET images were acquired using the established protocol and substantially free of artifacts.
Statistical Analyses
We used multivariate linear mixed effects (LME) models to describe the evolution of Alzheimer disease biomarkers. LME models have many benefits including providing a flexible approach to deal with an unequal number of measurement points or intervals. While neuroimaging analyses traditionally use univariate models, the field has begun using multivariate models which account for correlations between regional or voxelwise measurements30–32. Multivariate LME models can increased statistical power and reliability compared to univariate methods30,31. We implemented a Bayesian multivariate LME model to directly compare longitudinal biomarker changes across brain regions. Cortical and subcortical measurements were analyzed separately for each modality (PiB, FDG, and volumetric), resulting in total of six independent models.
The full Bayesian LME model is described in the supplemental material. Each region included fixed effects for mutation status, time from baseline, baseline EYO, and all possible two and three-way interactions. EYO was modeled as a restricted cubic spline with knots at the 0·10, 0·50, and 0·90 quantiles. We chose restricted cubic splines to model EYO as they represent a flexible approach for accounting for nonlinearities in the data without forcing any particular curve shape. Splines have also been used extensively in the literature to model longitudinal changes in Alzheimer disease biomarkers33,34. For every region we included random intercepts and slopes at the subject-level, as well as random intercepts for family affiliation. At the subject-level, covariance matrices were constructed so that intercepts and the slopes were allowed to correlate across all regions in a model.
To fit each model we used Stan (http://mc-stan.org/)35,36, an open source package for Hamilton Markov chain Monte Carlo analyses. A parameter, or combination of parameters, was considered statistically significant if the 99% equal-tailed credible intervals of the posterior distribution did not overlap zero. Analyses were run separately for each modality (MRI, PiB, and FDG). Within each modality one model simultaneously fit 34 cortical ROIs and a second model simultaneously fit 7 subcortical ROIs derived from FreeSurfer. Each regional comparison within a model is simply a different slice of the same multidimensional posterior distribution. The current analyses focus on the interaction between mutation status and the longitudinal rate of change. Including multiple regions within one model also allows for the direct comparison of rates of changes between regions (supplemental material).
Data sharing
Data from the DIAN project can be requested freely by researchers at the following website https://dian.wustl.edu/our-research/observational-study/dian-observational-study-investigator-resources/
Role of the funding source
The study sponsors had no role in the study design, data collection, data analysis, data interpretation, writing of the report, or the decision to submit the manuscript for publication. All coauthors had full access to the data in the study and the corresponding author had final responsibility for the decision to submit for publication.
Results
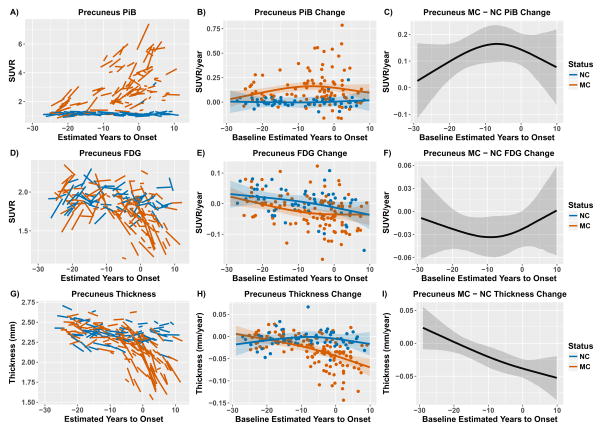
Population demographics are in Table 1. Subjects with longitudinal data had an average of 2·4 visits (sd 0·8) and 2·7 (sd 1·1) years of data. Figure 1 shows example LME model fits for one region. Figures depicting the model results for every ROI are available in supplemental materials. To avoid inadvertently revealing participants’ mutation status at the edges of our sample where there are only a few individuals, figures are displayed with baseline EYO −29 to +10.
Table 1.
Study demographics at baseline.
| Demographics at Baseline | |||
|---|---|---|---|
| Non-Carriers | Asymptomatic Carriers | Symptomatic Carriers | |
| Number | 148 | 141 | 88 |
| Females (%) | 85 (57%) | 78 (55%) | 49 (56%) |
| Age (years/sd) | 39·5 (11·4) | 34·6 (9·2) | 45·7 (9·9) |
| MMSE (mean/sd) | 29·0 (2·7) | 28·8 (2·7) | 23·9 (10·2) |
| CDR-SOB (mean/SD) | 0·0 (0·2) | 0·0 (0·1) | 3·6 (3·5) |
| EYO (years/sd) | −8·9 (11·4) | −13·7 (9·2) | 0·5 (7·1) |
| PSEN1/PSEN2/APP | 122/17/9 (82/11/6%) | 117/16/8 (83/11/6%) | 76/6/6 (86/7/7%) |
| N with Follow up (%) | 70 (47%) | 73 (52%) | 58 (66%) |
| N of visits*(sd) | 2·3 (0·8) | 2·3 (0·8) | 2·8 (1·2) |
| Follow up in years* (sd) | 3·0 (1·7) | 3·0 (1·6) | 2·0 (1·3) |
|
| |||
| Summary of Imaging Data | |||
|
| |||
| Data By Modality | PIB | FDG | MRI |
| 1 visit | 184 | 177 | 176 |
| 2 visits | 124 | 131 | 145 |
| 3 visits | 23 | 27 | 35 |
| 4 visits | 10 | 11 | 11 |
| 5 visits | 4 | 5 | 8 |
| 6 visits | 1 | 1 | 2 |
| Total Subjects | 346 | 352 | 377 |
Summary values are only for those individuals with longitudinal data
EYO - estimated years to dementia onset
MMSE – Mini Mental State Examination
CDR-SOB – Clinical Dementia Rating Sum of Boxes
Figure 1.
Modeling longitudinal change in the precuneus for PiB (top), FDG (middle), and cortical thickness (bottom). The left-hand panels (A, D, & G) depict the model estimates of longitudinal biomarkers. The middle panels (B, E, & H) depict the estimated rate of change across the course of the disease for mutation carriers and non-carriers. Individual random effect slope estimates are plotted as colored dots. The right hand panels (C, F, and I) depict the difference in rate of biomarker change between mutation carriers and non-carriers across the course of the disease. For both the middle and right-hand panels the shaded areas represent 99% credible intervals around the model estimates. The credible intervals are drawn from the actual distributions of model fits derived by the Hamilton Markov Chain Monte Carlos analyses. Any point in this difference curves where the shaded area is not touching the zero axis is a point in the disease progression (as measured by EYO) where the biomarker accumulation rate is different between groups. The first EYO point that was significantly different between groups was considered the initial diverge between groups. Figures depicting the model results for every ROI are available in supplemental materials. To avoid inadvertently revealing mutation status figures are displayed with baseline EYO −29 to +10.
The rate of Aβ accumulation is statistically higher in MC relative to NC participants starting more than two decades (EYO −22·2) before the expected age of dementia onset (Figure 1). As glucose utilization represents a natural biological property it contains both maturational and disease-related trajectories. In both groups, the precuneus FDG trajectories were initially positive, became neutral, and then negative. This negative directional acceleration begins earlier and was larger in MCs, with the rate of change becoming significantly less than NC at EYO −18·8. Finally, precuneus cortical thinning significantly differs in MC relative to NC at EYO −13·0. Supplemental material contains results for every ROI. Overall, in regions with a significant effect relative to NC, rates of Aβ deposition were significantly higher in MC at an average EYO of −18·9 (sd 3·3), metabolism began declining at an average EYO of −14·1 (sd 5·1), and MRI structural measures declined at an average EYO −4·7 (sd 4·2).
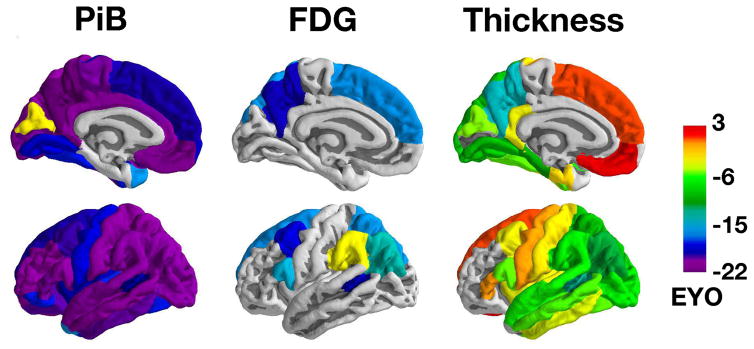
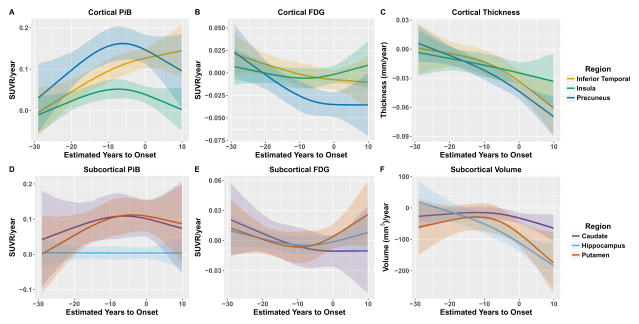
Figure 2 depicts EYOs when and whether the longitudinal rate of change first differs between MC and NC for each biomarker. The differences across regions and modalities reflect the temporal and spatial evolution of pathology over the course of the disease. Rates of biomarkers change in regions that are grey are never significantly different between groups. This information is presented in numeric form in Supplemental Tables 1 and 2. While many regions follow trajectories similar to the precuneus, the emergence of pathology varied throughout the brain. Further, there were regional differences by modality, for example, relative to NC the superior temporal lobe did not demonstrate a metabolic loss, but had atrophy changes at −5·6 EYO. Figure 3 depicts rates of change in MC for three cortical and three subcortical regions that exemplify common patterns.
Figure 2.
Emergence of neuroimaging biomarkers. The color scale represents the first point in the disease relative to estimated age at onset (EYO) where rates of biomarker change in that cortical region are significantly different between mutation carriers and non-carriers (akin to the first point where credible interval are different from zero in Figure 1 right panels). There is a temporal evolution where increased Aβ deposition precedes hypometabolism that in turn is followed by cortical thinning. Information for all modalities and regions is presented in numeric form in Supplemental Tables 1 and 2.
Figure 3.
Trajectories of biomarker accumulation in mutation carriers for three cortical (top) and three subcortical regions (bottom) for PiB (left), FDG (middle), and structural MRI (right) that highlight different patterns of change seen in different brain regions.
For PiB PET, 32/34 cortical regions showed significantly greater longitudinal rates of accumulation in MC relative to NC. The first point of divergence between groups varied across regions (EYO −22·2 to −2·5), with the precuneus, posterior cingulate gyrus, and medial orbital frontal cortex regions showing the earliest changes (~EYO −21). Of the 32 regions with significant differences, all but the cuneus (−2·5) occurred prior to an EYO of −15. In the seven subcortical regions the accumbens (−22·2), putamen (−17·0), and caudate (−16·4) demonstrated greater PiB accumulation rates in MC while the amygdala, hippocampus, palladium, and thalamus did not differ. Significant differences in progressive hypometabolism in MC relative to NC were less pronounced, with 8/34 cortical regions demonstrating significant interactions. The effects ranged from EYO −18·8 to −2·8, with the earliest effects detected in the precuneus, banks of the superior temporal sulcus, and caudal middle frontal cortex (EYO ~−18). No subcortical regions showed significant differences in the rate of FDG change. For MRI 24/34 cortical and 4/7 subcortical areas demonstrated increased rates of atrophy in MC relative to NC with effects appearing from EYO −13·0 to 2·3. The precuneus (−13·0), banks of the superior temporal sulcus (−11·5), and inferior parietal cortex (−10·6) demonstrated the earliest changes.
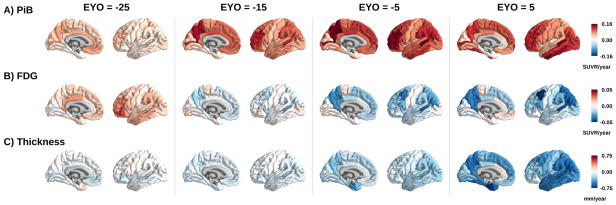
We also observed regional differences in the rates of biomarker change within the MC group. In the precuneus there was a rapid increase in Aβ deposition; this rate peaked but remained positive even after the predicted onset of dementia (Figure 3 and Figure 4). This was the most common pattern across areas. In other regions (e.g. insula) initial accelerations in Aβ deposition were followed by decelerations, leading to a plateau of total Aβ levels. In a subset of regions (e.g. inferior temporal cortex) the estimated rate of Aβ accumulation accelerates throughout the disease. Once declining, glucose metabolism in the precuneus showed prominent, worsening rates of hypometabolism before the rates stabilized (~ EYO −5), while in inferior temporal cortex the rate of metabolic loss modestly increased initially before quickly plateauing (Figure 3B). Many regions had relatively small rates of metabolic decline in MC, even at later EYOs. In regions with structural decline the trajectories were fairly consistent, with the rate of atrophy accelerating as the disease progressed. However, the absolute rate of decline was often different between regions. Matrices directly comparing the regional rates of change for each biomarker at different EYOs (−25, −15, −5, and 5) can be found in supplemental material. Voxel-wise movies depict the rate of change and total biomarker levels in MC at every EYO and the creation of these movies is detailed in supplemental material.
Figure 4.
Depictions of model estimates of rate of change in PiB (top), FDG (middle), and cortical thickness (bottom) in mutation carriers at an EYO of −25, −15, −5, and +5.
Discussion
AD is not static but possesses dynamism in terms of what pathological processes first appear, and how such pathology propagates throughout the brain. As dementia onset is predictable in ADAD, it provides an elegant model with which to examine pathological staging. Characterizing the spatial and temporal spread of pathology provides insight to the pathophysiology of the disease, informs how neuroimaging could aid optimal subject recruitment in clinical trials, and is critical to measure the efficacy of interventions on longitudinal biomarker measurements.
The primary goal of the current analysis was to find the first biomarker time point in the course of the disease where carriers of ADAD mutations demonstrated different rates of pathological progression relative-to non-carrier family members. This time point can be interpreted as the moment where longitudinal change in that brain area due to AD can first be detected with in vivo neuroimaging. The primary questions using this approach focused on regional differences across the brain within a marker (e.g. precuneus vs. parietal Aβ PET) as well as comparing spatial differences between biomarkers (e.g. Aβ PET vs. FDG PET).
Consistent with prior work we found that Aβ deposition was the first biomarker to demonstrate differences between mutation groups. MC had greater Aβ deposition more than 20 years before the expected age of symptom onset. Aβ increases were near ubiquitous, with most regions changing more than 14 years before the expected year of dementia. Measures of metabolism in ADAD represent overlapping maturational and disease changes. Both NC and MC cohorts had inverted U-shaped trajectories (Figure 1D & 1E), with the absolute levels of glucose metabolism initially modestly increasing with EYO, followed by a prolonged decrease. The key difference is that MC showed metabolic reductions earlier and to a greater degree than NC. While cross-sectional values still overlapped between groups early in the disease, longitudinal trajectories reveal divergence (supplementary material). The precuneus demonstrates the earliest metabolic decrease an EYO of −18·8, with significant regions on average becoming abnormal at EYO −14·1. Reductions in grey matter were the last neuroimaging biomarker to manifest and occured over the majority of the brain. Again the precuneus is one of the earliest regions to change, with declines emerging a decade before estimated dementia onset, while overall declines were most prolific in the five years preceding expected dementia onset. The direct comparison of the rates of biomarker change between regions is presented in supplementary material.
The relationships between the three biomarkers are complex. While all regions with metabolic decreases have abnormal Aβ accumulation, many regions with abnormal Aβ accumulation rates did not demonstrate elevated metabolic decline. Although FDG hypometabolism and structural decline are markers of degeneration, our results indicate they can be incongruent. In regions where they both occur, declines in glucose metabolism precede atrophy by ~5 to 10 years. However, there are regions that demonstrated β-amyloidosis and structural atrophy where significant metabolic decline was not detected (e.g. occipital and temporal regions). Portions of the medial temporal lobe (e.g. the hippocampus) did not manifest pathological change in Aβ or FDG, but had structural declines. Although there is generally a tripartite hierarchy such that β-amyloidosis precedes metabolic decline that in turn precede atrophy, these relationships are highly heterogeneous across the cortex.
Discordance between imaging biomarkers has been noted in sporadic AD 37–44. Due to the cross-sectional nature of the majority of the work, such spatial incongruences could be due to temporal lags in the emergence of pathologies.42,44 EYO, as a marker of disease time, is perfectly suited to detect such temporal evolutions. The current work does indeed clearly demonstrate that a temporal progression is present in some regions (e.g. PiB, FDG, and cortical thinning in the precuneus). However, despite the long disease window covered by the current study population, some region still only demonstrate a subset of pathologies. This suggests the incongruences are not simply a product of temporal lag, but can represent true heterogeneity. Other, unobserved, biomarkers such as those that measure tau pathology and inflammation, may help explain this heterogeneous relationship.
The current work presents the largest and most comprehensive analysis of neuroimaging data in ADAD to date. Still, the majority of longitudinal subjects had only a limited follow-up (average 2·4 visits); results at the edges of the EYO range where outliers have disproportional influence must be interpreted with care. There are also only modest numbers of subjects with PSEN2 and APP mutations. As the DIAN study gains more time points longitudinal estimates will be improved further and it may be possible to compare the three types of mutations. A greater number of individuals and time points will also increase the feasibility of modeling multiple modalities simultaneously across all brain regions as previously done using summary measures of pathology.45
The temporal and spatial ordering of biomarkers must also be interpreted with caveats. No one individual has data across the entire disease window, and our results represent population rather than individual subject effects. Further, as seen in regional fits (Figure 1 and supplemental material) some individuals differ from population trajectories. Thus, imaging data alone may not be sufficient to make individual-level disease stage predictions. Such predictions would require further work that accounts for individual differences due to factors such as genetic variability and lifestyle. The current work also utilizes partial volume corrected PET data,27,28 analyses without this step could have slightly different trajectories late in the disease.
The temporal ordering of biomarker change must also be viewed as relative rather than absolute. Our models are fit using a particular definition of EYO. Supplemental models using a modified definition of EYO indicate a preserved relative ordering (e.g. precuneus Aβ > hypometabolism > structural decline) but slight differences in absolute timing (e.g. shifts from EYO −22·2 to −19·8). Further, our results reflect the first detectable changes with PET and MRI, which are constrained by the inherent sensitivities and signal to noise properties of the imaging techniques. The current analyses utilize the cerebellum as a reference region for PET. Results using the brainstem instead were essentially unchanged (Supplementary Tables 3 and 4). Finally, although ADAD can serve as a model for sporadic AD, direct comparisons must explore potential differences.
Our results reveal complex patterns of biomarker accumulation across the brain. Elevations in β-amyloidosis occur more than two decades before and continue to accrue even after the expected year of symptom onset. Neurodegeneration measured with both FDG and structural MRI begins while Aβ is still increasing and occurs closer in time, but still well before the onset of dementia. While global measures likely capture a large degree of intraindividual variability, our results indicate not just when, but where pathology emerges in the brain. Understanding such longitudinal change provides insight into the pathophysiological progression of AD and has implications for clinical trials.
Supplementary Material
Research in context.
Evidence before this study
Using PubMed and Google Scholar the authors reviewed prior work on longitudinal neuroimaging markers of Alzheimer pathology with a focus on autosomal dominant Alzheimer disease (ADAD). We searched for all articles prior to October 31st, 2017 with no language restrictions for the keywords Alzheimer’s, Alzheimer, longitudinal, positron emission tomography, PET, MRI, atrophy, FDG, hypometabolism, familial, and autosomal. Theories proposed initially in 2010 by Jack and colleagues and revised in 2013 posited temporal trajectories of Alzheimer biomarkers relative to each other and clinical decline. Work by Bateman and colleagues in 2012, Benzinger and colleagues in 2013, and Fleisher and colleagues in 2015 depict such temporal ordering of biomarkers in ADAD populations derived from cross-sectional analyses. There was also a small subset of longitudinal ADAD studies, but these had one or more limitation such as small populations (n<50), examination of only one biomarker, not accounting for regional differences or correlations in the brain, or had a short duration of longitudinal followup.
Added value of this study
Our study presents the first known work examining both the longitudinal temporal trajectories and spatial patterns of Alzheimer pathology in ADAD cohorts using neuroimaging. This work also presents the largest known cohort to date of ADAD individuals studied longitudinally with multiple neuroimaging biomarkers. Longitudinal analyses can provide a more accurate and powerful way to model the temporal emergence of pathology in ADAD. We find that mutation carriers first display Aβ accumulation, followed by hypometabolism, and finally structural atrophy; this is consistent with theoretical models and cross-sectional estimates from ADAD. Most importantly we consider such temporal relationships not in one singular summary measure, but characterize these trajectories throughout the brain. We found that the accrual of pathology varied throughout the brain and by modality in terms of the time of initial emergence and the rates of longitudinal change. These findings suggest region specific vulnerabilities to β-amyloidosis, metabolic decline, and atrophy that change over the course of the disease.
Implications of all the available evidence
Our results build upon existing evidence characterizing biomarkers in clinical and preclinical Alzheimer disease. Our findings suggest that imaging biomarkers follow a sequential pattern, with β-amyloidosis, hypometabolism, and structural atrophy emerging more than twenty, fifteen, and ten years respectively before the expected onset of dementia. Although there is a general hierarchical pattern, there was considerable regional heterogeneity. Most commonly, regions demonstrated an increase in β-amyloidosis and structural atrophy, but there was not evidence of metabolic declines. Further, rather than being homogenous, the same biomarker often demonstrates different longitudinal trajectories across brain regions. Characterizing the temporal and regional dynamics provides insight into disease pathophysiology. This information is critical to decide how to best use neuroimaging biomarkers in clinical trials for subject selection as well as outcomes measures.
Acknowledgments
Funding
National Institutes of Health UFAG032438, UL1TR000448, P30NS098577, R01EB009352, and NS080675, the German Center for Neurodegenerative Diseases, and the Medical Research Council Dementias Platform UK (MR/L023784/1 and MR/009076/1). ClinicalTrials.gov number, NCT00869817
Foremost we wish to acknowledge the dedication of the participants and their families, whom without these studies would not be possible. We additionally thank all of the participating researchers in the Dominantly Inherited Alzheimer Network. This research was funded by the National Institutes of Health (NIH) UFAG032438, UL1TR000448, P30NS098577, R01EB009352, the German Center for Neurodegenerative Diseases (DZNE), the National Institute for Health Research (NIHR) Queen Square Dementia Biomedical Research Centre, and the Medical Research Council Dementias Platform UK (MR/L023784/1 and MR/009076/1). DIAN ClinicalTrials.gov number, NCT00869817. We acknowledge the financial support of Fred Simmons and Olga Mohan, the Barnes-Jewish Hospital Foundation, the Charles F. and Joanne Knight Alzheimer’s Research Initiative, the Hope Center for Neurological Disorders, the Mallinckrodt Institute of Radiology and the Paula and Rodger Riney fund. Computations were performed using the facilities of the Washington University Center for High Performance Computing, which were partially funded by NIH grants 1S10RR022984-01A1 and 1S10OD018091-01.
Footnotes
Contributors
BAG and TMB equally contributed to the present work and wrote the manuscript, analyzed the data, and generated the figures and movies. YS, AH, AD, SF, JC, CRJ, and MMW oversaw data quality control and processing. CX, NJC, JH, DSM, AMF, DMH, RCH, KLP, EM, GW, MER, JCM, RJB, and TLSB and oversaw overall study design and general implementation. RJB and TLSB assisted in data interpretation. BMA, SBB, AMB, DMC, JPC, SC, StF, NCF, NRG, CF, JL, CLM, MNR, SS, AJS, PRS, and PMT oversaw study implementation and data collection at their respective institutions. All authors revised the manuscript.
Declarations of Interest
BAG and BMA report participating in a clinical trial of AV-1451 sponsored by Avid Radiopharmaceuticals. EM reports grants from Dominantly Inherited Alzheimer Network Trials Unit Pharma Consortium, outside the submitted work. CX reports grants from the NIA outside the submitted work. JH reports personal fees from Biogen and Lundbeck, outside the submitted work. CRJ reports consulting services for Lilly Co. and grants from NIH, outside the submitted work. DSM reports grants from the NIH outside and support from Radiologics, Inc., both outside the conduct of the study. AMF reports personal fees from DiamiR, personal fees from LabCorp, personal fees from IBL International, personal fees from Genentech, grants from Roche Diagnostics, grants from Fujirebio, grants from Biogen, outside the submitted work. DMC reports grants from Alzheimer’s Society, during the conduct of the study. JL reports grants from German Ministry of Reseach and Education, during the conduct of the study. AJS reports non-financial support from Avid Radiopharmaceuticals and grants from Eli Lilly, outside the submitted work. SBB reports grants from NIH, during the conduct of the study; other from Lundbeck, other from Grifols Biologicals, outside the submitted work. MNR reports support from Servier and Merck outside the submitted work. NCF reports personal fees from Janssen, Roche/Genentech, Janssen Alzheimer’s Immunotherapy, Eli Lilly, Novartis Pharma AG, Sanofi GSK, and Biogen, outside the submitted work. NRG reports Eli Lilly Multi center Treatment Study Grant, Biogen Multi center Treatment Study Grant, and Cytox consultation. PRS reports grants from NIH/NIA, the Anonymous Foundation, the Mason Foundation, from Roth Charitable Foundation during the conduct of the study; personal fees from ICME Speakers & Entertainers, outside the submitted work; and serving as the Interim Director of the Australian National Health and Medical Research Council (NHMRC). DMH co-founded and is on the scientific advisory board of C2N Diagnostics. DMH is an inventor on a submitted patent “Antibodies to Tau” that is licensed by Washington University to C2N Diagnostics. This patent was subsequently licensed to AbbVie. DMH is an inventor on patents licensed by Washington University to Eli Lilly and Company based on intellectual property related to the anti-Abeta antibody solanezumab. DMH consults for Genentech, AbbVie, Eli Lilly, GlaxoSmithKline, Proclara Biosciences, and Denali. MMW reports grants from NIH/NIA/NIMH, grants from DOD, grants from CA Dept. of Public Health, grants and other from Alzheimer’s Drug Discovery Foundation (ADDF), grants from Larry L. Hillblom Foundation, grants from PCORI, grants from Global Alzheimer’s Platform Foundation, grants from Monell Chemical Senses Center, grants and other from Alzheimer’s Association, other from Pfizer, other from Alzheon, Inc., other from Eli Lilly, other from Dolby Ventures, other from ADNI, other from MRI Magazine, other from Alzheimer’s & Dementia Magazine, other from Synarc, other from Janssen, other from Accera Pharma, other from Avid Radiopharma, other from Araclon, other from Merck, other from Scienomics Group, other from AVOS Consulting, other from INC Research, other from Biogen Idec, other from BioClinica, other from Howard University, other from Guidepoint, other from GLG Research, other from Genentech, other from Alzeca, outside the submitted work. PMT reports grants from NIA, NIBIB, and NINDS outside of the submitted work. JCM reports grants from NIH grant P50AG005681, grants from NIH grant P01AG003991, grants from NIH grant P01AG026276, grants from NIH grant UF01AG032438, during the conduct of the study; other from Lilly USA, outside the submitted work. RJB reports grants from NIH/NIA U19AG32438 and an Anonymous Foundation, during the conduct of the study, grants from Eli Lilly, Roche, Pharma Consortium (Abbvie, AstraZeneca, Biogen, Eisai, Eli Lilly and Co., Hoffman La-Roche Inc., Janssen, Pfizer, Sanofi-Aventi), and Tau SILK/PET Consortium (Biogen/Abbvie/Lilly), non-financial support from Avid Radiopharmaceuticals, personal fees and other from Washington University, personal fees and non-financial support from Roche, IMI, FORUM, and Pfizer, and personal fees from Merck, Johnson and Johnson, outside the submitted work. TLSB reports grants, non-financial support and other from Avid Radiopharmaceuticals/Eli Lilly, other from Roche, outside the submitted work. TMB, YS, AH, AD, SF, JC, GW, NJC, RCH, KLP, AMB, JPC, SC, StF, CF, CLM, SS, and MER report no conflicts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pike KE, Savage G, Villemagne VL, et al. β-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain. 2007:130. doi: 10.1093/brain/awm238. [DOI] [PubMed] [Google Scholar]
- 2.Hardy JA, Higgins GA. Alzheimer’s disease: The amyloid cascade hypothesis. Science (80-) 1992;256:184–5. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 3.Jack CR, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–16. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moulder KL, Snider BJ, Mills SL, et al. Dominantly Inherited Alzheimer Network: facilitating research and clinical trials. Alzheimers Res Ther. 2013;5:48. doi: 10.1186/alzrt213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryman DC, Acosta-Baena N, Aisen PS, et al. Symptom onset in autosomal dominant Alzheimer disease: a systematic review and meta-analysis. Neurology. 2014;83:253–60. doi: 10.1212/WNL.0000000000000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bateman RJ, Xiong C, Benzinger TLS, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benzinger TLS, Blazey T, Jack CR, et al. Regional variability of imaging biomarkers in autosomal dominant Alzheimer’s disease. Proc Natl Acad Sci U S A. 2013;110:E4502–4509. doi: 10.1073/pnas.1317918110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleisher AS, Chen K, Quiroz YT, et al. Associations between biomarkers and age in the presenilin 1 E280A autosomal dominant Alzheimer disease kindred: a cross-sectional study. JAMA Neurol. 2015;72:316–24. doi: 10.1001/jamaneurol.2014.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yau W-YW, Tudorascu DL, McDade EM, et al. Longitudinal assessment of neuroimaging and clinical markers in autosomal dominant Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 2015;14:804–13. doi: 10.1016/S1474-4422(15)00135-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weston PSJ, Nicholas JM, Lehmann M, et al. Presymptomatic cortical thinning in familial Alzheimer disease: A longitudinal MRI study. Neurology. 2016 doi: 10.1212/WNL.0000000000003322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang F, Gordon BA, Ryman DC, et al. Cerebral amyloidosis associated with cognitive decline in autosomal dominant Alzheimer disease. Neurology. 2015 doi: 10.1212/WNL.0000000000001903. published online Aug 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sala-Llonch R, Llad? A, Fortea J, et al. Evolving brain structural changes in PSEN1 mutation carriers. Neurobiol Aging. 2015;36:1261–70. doi: 10.1016/j.neurobiolaging.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 13.Schott JM, Fox NC, Frost C, et al. Assessing the onset of structural change in familial Alzheimer’s disease. Ann Neurol. 2003;53:181–8. doi: 10.1002/ana.10424. [DOI] [PubMed] [Google Scholar]
- 14.Fagan AM, Xiong C, Jasielec MS, et al. Longitudinal change in CSF biomarkers in autosomal-dominant Alzheimer’s disease. Sci Transl Med. 2014;6:226ra30. doi: 10.1126/scitranslmed.3007901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knight WD, Kim LG, Douiri A, Frost C, Rossor MN, Fox NC. Acceleration of cortical thinning in familial Alzheimer’s disease. Neurobiol Aging. 2011;32:1765–73. doi: 10.1016/j.neurobiolaging.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Kinnunen KM, Cash DM, Poole T, et al. Presymptomatic atrophy in autosomal dominant Alzheimer’s disease: a serial MRI study. Alzheimer’s Dement. 2017 doi: 10.1016/j.jalz.2017.06.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson WK, Hallmayer J, O’Hara R Initiative the ADN. Design Considerations for Characterizing Psychiatric Trajectories Across the Lifespan: Application to Effects of APOE-ε4 on Cerebral Cortical Thickness in Alzheimer’s Disease. Am J Psychiatry. 2011;168:894–903. doi: 10.1176/appi.ajp.2011.10111690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu Z, Shen X, Pan W, et al. Longitudinal analysis is more powerful than cross-sectional analysis in detecting genetic association with neuroimaging phenotypes. PLoS One. 2014;9:e102312. doi: 10.1371/journal.pone.0102312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mills SM, Mallmann J, Santacruz AM, et al. Preclinical trials in autosomal dominant AD: implementation of the DIAN-TU trial. Rev Neurol (Paris) 2013;169:737–43. doi: 10.1016/j.neurol.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reiman EM, Langbaum JBS, Fleisher AS, et al. Alzheimer’s Prevention Initiative: a plan to accelerate the evaluation of presymptomatic treatments. J Alzheimers Dis. 2011;26(Suppl 3):321–9. doi: 10.3233/JAD-2011-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sperling RaA, Rentz DMM, Johnson KaA, et al. The A4 Study: Stopping AD before Symptoms Begin? Sci Transl Med Med. 2014;6:228fs13–228fs13. doi: 10.1126/scitranslmed.3007941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–4. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 23.Jack CCR, Bernstein MA, Borowski BBJ, et al. Update on the magnetic resonance imaging core of the Alzheimer’s disease neuroimaging initiative. Alzheimer’s Dement. 2010;6:212–20. doi: 10.1016/j.jalz.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischl B. FreeSurfer. Neuroimage. 2012;62:774–81. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fischl B, Dale AMM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–5. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su Y, D’Angelo GM, Vlassenko AG, et al. Quantitative Analysis of PiB-PET with FreeSurfer ROIs. PLoS One. 2013;8:e73377. doi: 10.1371/journal.pone.0073377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Su Y, Blazey TM, Snyder AZ, et al. Partial volume correction in quantitative amyloid imaging. Neuroimage. 2015;107:55–64. doi: 10.1016/j.neuroimage.2014.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rousset OG, Ma Y, Evans AC. Correction for partial volume effects in PET: Principle and validation. J Nucl Med. 1998;39:904–11. [PubMed] [Google Scholar]
- 29.Joshi A, Koeppe RA, Fessler JA. Reducing between scanner differences in multi-center PET studies. Neuroimage. 2009;46:154–9. doi: 10.1016/j.neuroimage.2009.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bernal-Rusiel JL, Reuter M, Greve DN, Fischl B, Sabuncu MR. Spatiotemporal linear mixed effects modeling for the mass-univariate analysis of longitudinal neuroimage data. Neuroimage. 2013;81:358–70. doi: 10.1016/j.neuroimage.2013.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernal-Rusiel JL, Greve DN, Reuter M, Fischl B, Sabuncu MR. Statistical analysis of longitudinal neuroimage data with Linear Mixed Effects models. Neuroimage. 2013;66:249–60. doi: 10.1016/j.neuroimage.2012.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bilgel M, Prince JL, Wong DF, Resnick SM, Jedynak BM. A multivariate nonlinear mixed effects model for longitudinal image analysis: Application to amyloid imaging. Neuroimage. 2016;134:658–70. doi: 10.1016/j.neuroimage.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jack CR, Wiste HJ, Lesnick TG, et al. Brain β-amyloid load approaches a plateau. Neurology. 2013;80:890–6. doi: 10.1212/WNL.0b013e3182840bbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jack CR, Vemuri P, Wiste HJ, et al. Shapes of the trajectories of 5 major biomarkers of Alzheimer disease. Arch Neurol. 2012;69:856–67. doi: 10.1001/archneurol.2011.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carpenter B, Lee D, Brubaker MA, et al. Stan: A Probabilistic Programming Language. J Stat Softw. 2016 doi: 10.18637/jss.v076.i01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gelman A, Lee D, Guo J. Stan: A Probabilistic Programming Language for Bayesian Inference and Optimization. J Educ Behav Stat. 2015;40:530–43. [Google Scholar]
- 37.Gordon BA, Blazey T, Benzinger TL. Regional variability in Alzheimer’s disease biomarkers. Future Neurol. 2014;9:131–4. doi: 10.2217/fnl.14.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.La Joie R, Perrotin A, Barré L, et al. Region-specific hierarchy between atrophy, hypometabolism, and β-amyloid (Aβ) load in Alzheimer’s disease dementia. J Neurosci. 2012;32:16265–73. doi: 10.1523/JNEUROSCI.2170-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grothe MJ, Teipel SJ. Spatial patterns of atrophy, hypometabolism, and amyloid deposition in Alzheimer’s disease correspond to dissociable functional brain networks. Hum Brain Mapp. 2016;37:35–53. doi: 10.1002/hbm.23018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edison P, Archer HA, Hinz R, et al. Amyloid, hypometabolism, and cognition in Alzheimer disease: an [11C]PIB and [18F]FDG PET study. Neurology. 2007;68:501–8. doi: 10.1212/01.wnl.0000244749.20056.d4. [DOI] [PubMed] [Google Scholar]
- 41.Lehmann M, Ghosh PM, Madison C, et al. Diverging patterns of amyloid deposition and hypometabolism in clinical variants of probable Alzheimer’s disease. Brain. 2013;136:844–58. doi: 10.1093/brain/aws327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Förster S, Grimmer T, Miederer I, et al. Regional Expansion of Hypometabolism in Alzheimer’s Disease Follows Amyloid Deposition with Temporal Delay. Biol Psychiatry. 2012;71:792–7. doi: 10.1016/j.biopsych.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 43.Alexopoulos P, Kriett L, Haller B, et al. Limited agreement between biomarkers of neuronal injury at different stages of Alzheimer’s disease. Alzheimer’s Dement. 2014;10:684–9. doi: 10.1016/j.jalz.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 44.Förster S, Yousefi BH, Wester H-J, et al. Quantitative longitudinal interrelationships between brain metabolism and amyloid deposition during a 2-year follow-up in patients with early Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2012;39:1927–36. doi: 10.1007/s00259-012-2230-9. [DOI] [PubMed] [Google Scholar]
- 45.Donohue MC, Jacqmin-Gadda H, Le Goff M, et al. Estimating long-term multivariate progression from short-term data. Alzheimer’s Dement. 2014;10:S400–10. doi: 10.1016/j.jalz.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.