Abstract
Objectives
Left-sided methicillin-susceptible Staphylococcus aureus (MSSA) endocarditis treated with cloxacillin has a poorer prognosis when the vancomycin minimum inhibitory concentration (MIC) is ≥1.5 mg/L. We aimed to validate this using the International Collaboration on Endocarditis cohort and to analyse whether specific genetic characteristics were associated with a high vancomycin MIC (≥1.5 mg/L) phenotype.
Methods
All patients with left-sided MSSA infective endocarditis treated with antistaphylococcal β-lactam antibiotics between 2000 and 2006 with available isolates were included. Vancomycin MIC was determined by Etest as either high (≥1.5 mg/L) or low (<1.5 mg/L). Isolates underwent spa typing to infer clonal complexes and multiplex PCR for identifying virulence genes. Univariate analysis was performed to evaluate the association between in-hospital and 1-year mortality, and vancomycin MIC phenotype.
Results
Sixty-two cases met the inclusion criteria. Vancomycin MIC was low in 28 cases (45%) and high in 34 cases (55%). No significant differences in patient demographic data or characteristics of infection were observed between patients with infective endocarditis due to high and low vancomycin MIC isolates. Isolates with high and low vancomycin MIC had similar distributions of virulence genes and clonal lineages. In-hospital and 1-year mortality did not differ significantly between the two groups (32% (9/28) vs. 27% (9/34), p 0.780; and 43% (12/28) vs. 29% (10/34), p 0.298, for low and high vancomycin MIC respectively).
Conclusions
In this international cohort of patients with left-sided MSSA endocarditis treated with antistaphylococcal β-lactams, vancomycin MIC phenotype was not associated with patient demographics, clinical outcome or virulence gene repertoire.
Keywords: Endocarditis, Genotype, Phenotype, Staphylococcus aureus, Vancomycin MIC
Introduction
The impact of a high vancomycin minimum inhibitory concentration (MIC) phenotype (HVM; >1.5 mg/L) in methicillin-susceptible Staphylococcus aureus (MSSA) bacteraemia and infective endocarditis (IE) is poorly known. To date several studies have reported higher rates of complications and mortality in patients with MSSA bacteraemia caused by strains with HVM [1,2], as well as a correlation with agr dysfunction and agr type II polymorphism [3]. Nonetheless, a more recent study did not find significant differences on agr subgroup and function according to vancomycin MIC [4]. The association between HVM and significantly higher mortality was also demonstrated in a Spanish a cohort of 93 patients with MSSA IE treated with cloxacillin [5].
We aimed to explore the association between high vancomycin MIC and clinical outcome among patients with left-sided MSSA IE in the International Collaboration Endocarditis (ICE) Cohort. We also aimed to study whether HVM was identifiable by a genetic signature of specific polymorphisms and virulence factors.
Methods
Database
The ICE prospective cohort study has been described previously [6]. All patients were included from sites that met performance criteria for participation, and the strains obtained from IE episodes were available in the ICE Microbiology Repository [7]. All participating sites had institutional review board or ethical committee approval or a waiver, and informed consent was obtained from all patients according to local standards as required by the coordinating centre (Duke University Medical Center).
Study sample
Patients and settings
IE isolates were obtained from the ICE Microbiology Repository [7]. Subjects in the ICE Microbiology Repository cohort with available frozen bloodstream isolates with definite MSSA IE treated with β-lactams were eligible for inclusion in this study. Seventeen of these strains were included in the Spanish cohort [5]. All patients who survived had at least 1 year’s follow-up.
Definitions
IE was defined according to the modified Duke criteria [8] and was considered to be left-sided if no right-sided (tricuspid or pulmonary valve) vegetations were present on echocardiographic examination, surgery or autopsy. The rest of the definitions have been provided in detail elsewhere [9].
Geographic regions
Twenty-five sites from a variety of geographic regions participated in the study (Supplementary data).
Microbiologic methods
ICE methodology for microbiologic procedures has been defined elsewhere [6,10]. Detailed microbiologic methods can be found in the Supplementary data.
Statistical analysis
Categorical variables were expressed as percentages and compared by Fisher’s exact test. Continuous variables were expressed as means or medians and compared by nonparametric tests. Survival analysis was performed by Kaplan-Meier analysis, and curves were compared by the log-rank test. A two-sided p value of <0.05 was considered statistically significant.
Results
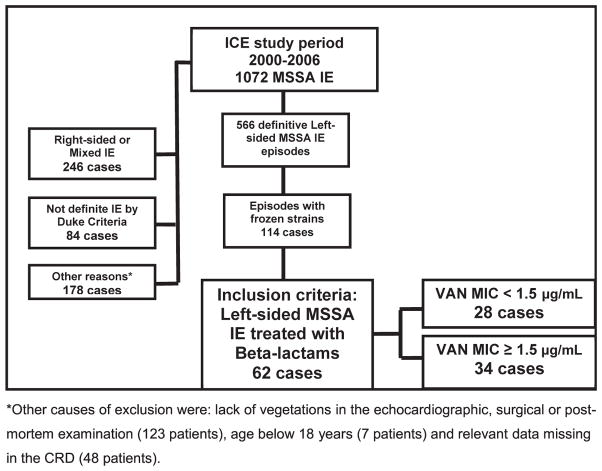
The study flowchart is provided in Fig. 1.
Fig. 1.
Flowchart of patients included in study. ICE, International Collaboration on Endocarditis; IE, infective endocarditis; MIC, minimum inhibitory concentration; MSSA, methicillin-susceptible Staphylococcus aureus; VAN, vancomycin.
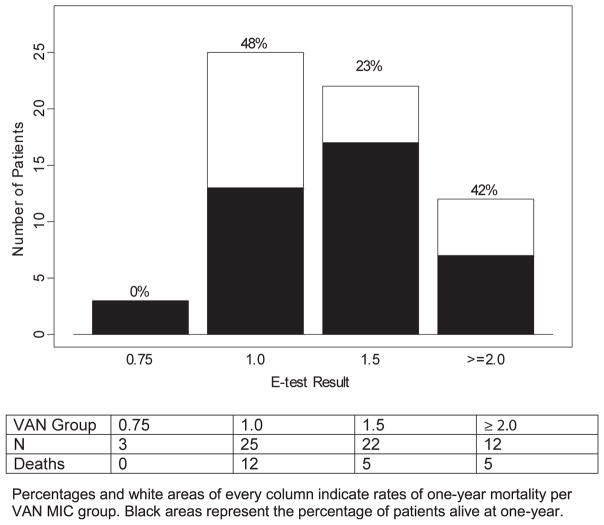
The distribution of vancomycin MIC among the 62 cases included in the study is displayed in Fig. 2, along with the respective rates of mortality.
Fig. 2.
Distribution of vancomycin MIC within cohort of 62 left-sided MSSA infective endocarditis and overall 1-year mortality rates according to vancomycin MIC determination. MIC, minimum inhibitory concentration.
Demographics and clinical characteristics of patients with low vancomycin MIC (LVM) and HVM are shown in Table 1.
Table 1.
Demographics, clinical characteristics and outcomes of 62 episodes of left-sided MSSA IE treated with β-lactams according to high or low vancomycin MIC
| Characteristic | Vancomycin MIC <1.5 μg/mL (n = 28) | Vancomycin MIC ≥1.5 μg/mL (n = 34) | p |
|---|---|---|---|
| Age, years, mean (SD) | 61.1 (18.5) | 60.1 (14.3) | 0.396 |
| Male gender, n (%) | 19 (68%) | 27 (79%) | 0.386 |
| Type of endocarditis | 0.548 | ||
| Native valve | 23 (82%) | 25 (74%) | |
| Prosthetic valve | 5 (19%) | 9 (27%) | |
| Origin of acquisition | 0.916 | ||
| Community acquired | 17 (61%) | 23 (68%) | |
| Nosocomial | 8 (29%) | 9 (26%) | |
| Healthcare related | 2 (7%) | 2 (6%) | |
| Unknown | 1 (3%) | 0 | |
| Geographic area | 0.333 | ||
| North America | 4 (14%) | 10 (29%) | |
| Europe/Mideast | 20 (71%) | 22 (65%) | |
| South America | 1 (4%) | 0 | |
| Australia/New Zealand | 3 (11%) | 2 (6%) | |
| Complications | |||
| Heart failure | 13 (46%) | 10 (29%) | 0.195 |
| Systemic emboli | 10 (36%) | 8 (23%) | 0.400 |
| Stroke | 7 (25%) | 9 (27%) | 1.000 |
| Paravalvular complications | 8 (29%) | 11 (32%) | 0.788 |
| New conduction abnormality | 0 (–) | 3 (9%) | 0.245 |
| Persistent bacteraemia | 4 (14%) | 4 (12%) | 1.000 |
| Surgical treatment | 9 (32%) | 14 (41%) | 0.599 |
| Relapsea | 0 | 0 | 1.000 |
| Mortality | |||
| In-hospital mortality | 9 (32%) | 9 (27%) | 0.780 |
| 1-year mortality | 12 (43%) | 10 (29%) | 0.298 |
IE, infective endocarditis; MIC, minimum inhibitory concentration; MSSA, methicillin-susceptible Staphylococcus aureus.
Defined as new episode of endocarditis due to the same microorganism that caused the first IE within next 12 months.
Complications, surgical rates and mortality according to vancomycin MIC groups are shown in Table 1. Differences between the two groups did not reach statistical significance for any of the variables. For LVM, in-hospital and 1-year mortality were 32% (9/ 28) and 43% (12/28) respectively, while in-hospital mortality was 27% (9/34) and 1-year mortality 29% (10/34) for HVM.
Differences in genotypic characteristics between the two groups according to MIC phenotypes are shown in the Supplementary data. No differences were detected regarding adhesins, toxins or other putative virulence factors.
The univariate analysis of risk factors for 1-year mortality is shown in Table 2. HVM, type of agr and clonal complexes (CC) were not significantly associated with mortality. The analysis for inhospital mortality using the same variables did not differ from that of 1-year mortality (data not shown).
Table 2.
Bivariate analysis of risk factors for 1-year mortalitya
| Variable | Alive (n = 39) | Dead (n = 22) | OR (95% CI) | p |
|---|---|---|---|---|
| Demographic data | ||||
| Age >65 years | 16 (40%) | 11 (50%) | 1.5 (0.5–4.9) | 0.593 |
| Male sex | 31 (78%) | 15 (68%) | 0.62 (0.2–2.4) | 0.546 |
| Prosthetic valve IE | 11 (29%) | 3 (14%) | 0.41 (0.1–1.9) | 0.338 |
| Diabetes | 8 (20%) | 4 (18%) | 0.89 (0.2–3.9) | 1.000 |
| Community acquisition | 26 (65%) | 14 (64%) | 1.76 (0.0–141.9) | 0.889 |
| North America | 8 (20%) | 6 (27%) | 1.77 (0.1–143.1) | 0.444 |
| Clinical features | ||||
| Paravalvular complications | 8 (20%) | 11 (50%) | 4.0 (1.1–14.6) | 0.021 |
| Stroke | 6 (15%) | 10 (46%) | 4.72 (1.2–19.1) | 0.015 |
| Heart failure | 10 (46%) | 10 (46%) | 1.73 (0.5–5.7) | 0.411 |
| Persistent bacteraemia | 5 (13%) | 3 (14%) | 1.11 (0.2–6.4) | 1.000 |
| Surgery | 14 (35%) | 9 (41%) | 1.29 (0.4–4.2) | 0.785 |
| Microbiologic features | ||||
| Vancomycin MIC ≥1.5 mg/L | 24 (60%) | 10 (45%) | 0.56 (0.2–1.8) | 0.298 |
| CC30 | 7 (18%) | 6 (27%) | 1.77 (0.4–7.3) | 0.516 |
| CC8 | 1 (3%) | 3 (14%) | 6.16 (0.4–331.3) | 0.546 |
| CC15 | 6 (15%) | 0 | 0.0 (0–1.1) | 0.081 |
| agrI | 15 (38%) | 7 (32%) | 0.78 (0.2–2.6) | 0.784 |
| agrII | 13 (33%) | 6 (27%) | 0.78 (0.2–2.7) | 0.777 |
| agrIII | 11 (28%) | 9 (41%) | 1.83 (0.5–6.2) | 0.394 |
| see | 8 (20%) | 10 (46%) | 3.33 (0.9–12.2) | 0.044 |
| sei | 33 (83%) | 22 (100%) | 2.94 (1.1–13.7) | 0.044 |
| chp | 39 (98) | 16 (73%) | 0.07 (0.0–0.7) | 0.006 |
| eta | 8 (20%) | 6 (27%) | 1.5 (0.4–5.9) | 0.539 |
| pvl | 4 (10%) | 5 (23%) | 2.65 (0.5–14.9) | 0.259 |
CC, clonal complex; CI, confidence interval; IE, infective endocarditis; MIC, minimum inhibitory concentration; OR, odds ratio.
Analysis for in-hospital mortality using the same variables did not differ from that of 1-year mortality (data not shown).
The Kaplan-Meier survival plot at 1 year according to vancomycin MIC group is provided in the Supplementary data.
Discussion
Vancomycin MIC was not associated with complications or mortality among patients with MSSA left-sided IE treated with β-lactams. Thus, this study was unable to validate findings from the study of Cervera et al. [5], from which two main hypotheses were raised: first, HVM in MSSA isolates was associated with higher mortality in patients with left-sided IE as a result of an increased rate of major embolic events; and second, a genomic signature identified MSSA isolates with HVM.
The main findings from studies investigating the relationship between vancomycin MIC and prognosis of MSSA and IE, as well as its relationship with agr dysfunction, are shown in Table 3.
Table 3.
Summary of main findings from studies assessing the relationship between vancomycin MIC and prognosis in MSSA bacteraemia and left-sided IE
| Study | Design | SAB/IE (n) | Overall mortality | Genetic factors | Main outcomes analysis | |
|---|---|---|---|---|---|---|
|
| ||||||
| Low VAN MIC (<1.5 μg/mL) | High VAN MIC (≥1.5 μg/mL) | |||||
| Kalil, 2014 [11] | Systematic review and metaanalysis | SAB (8291, both MRSA and MSSA) | 25.8% (1430/5551) | 26.8% (734/2740) | NA | RD 1.6% (95% CI −2.3 to 5.6); p 0.43 (for absolute risk of mortality, combining 30-day mortality and in- hospital mortality) |
| Holmes, 2011 [1] | Prospective multicentre cohort study | SAB (532; 266 of which MSSA treated with β-lactams only) | 12.2% (24/193) | 26.8% (18/68) | NA | p 0.011 (for 30-day mortality) |
| Holmes, 2014 [12] | Analysis of a subset of strains from [1] | SAB (252 MSSA isolates) | NA | NA | Associated to HVM: CC8, agr dysfunction, agr genotype II, blaZ, sea, clfA, splA and ACME locus Associated to LVM: CC22, CC88 and CC188 | Associated to HVM: CC8 p <0.001), agr dysfunction (p 0.014), agr genotype II (p 0.043), blaZ (p 0.002), sea (p <0.001), clfA (p <0.001), splA (p <0.001) and ACME locus (p 0.02). Associated to LVM: CC22 (p <0.001), CC88 (p <0.001) and CC188 (p 0.002) |
| Aguado, 2011 [2] | Retrospective, single-centre cohort | Catheter- related SAB (99, all MSSA) | 10.5% (8/76) | 26.1% (6/23) | NA | p 0.13 (for 30-day mortality) OR = 22.9, (95% CI 6.7 to 78.1) for complicated SAB |
| López-Cortés, 2015 [4] | Prospective, single-centre cohort | SAB (135, all MSSA) | 23.6% (25/106) | 10.3% (3/29) | No differences in agr distribution or absence of δ-haemolysin between isolates with HVM and those with LVM. HVM was not more frequent in specific clones | RR = 0.44 (95% CI 0.14 to 1.35) for 14-day mortality |
| Viedma, 2014 [3] | Retrospective, single-centre cohort | SAB (84, all MSSA) | 24.1%, (7/29) | 45.5% (25/55) | HVM: agr II polymorphism: 17.2% ; average levels of RNAIII gene expression: ΔCt 1.5 ± 2.11 LVM: agr II polymorphism: 41.8% ; average levels of RNAIII gene expression: ΔCt 4.05 ± 3.29 |
In-hospital mortality: p 0.057 agr dysfunction: p 0.023. RNAIII expression: p <0.01 |
| Cervera, 2014 [5] | Prospective, single-centre cohort | MSSA IE (93) | 31% (16/53) | 53% (21/40) | NA | In-hospital mortality: p 0.035; Patients with HVM presented significantly more severe embolic events |
| Current study | Prospective, multicentre cohort | MSSA IE (62) | 32% (9/28) | 27% (9/34) | HVM: agrII polymorphism: 19% LVM: agrII polymorphism: 38% |
In-hospital mortality: p 0.780. agrII polymorphism: p 0.157 |
CI, confidence interval; HVM, high vancomycin MIC; IE, infective endocarditis; LVM, low vancomycin MIC; MIC, minimum inhibitory concentration; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible S. aureus; NA, not addressed; OR, odds ratio; RD, relative difference; RR, relative risk; SAB, S. aureus bacteraemia; VAN, vancomycin.
Higher mortality among patients with LVM than in patients with HVM was found in the present study, as well as higher rates of systemic embolic events (36% vs. 23%) and persistent bacteraemia (14% vs.12%), neither of which reached statistical significance. Given that no significant differences were found in virulence factors between the two groups, we might speculate that in this data set, as a result of the high proportion of patients with MSSA strains harbouring LVM and CC30, a high rate of detected and undetected haematogenous complications in the LVM may explain the higher mortality in this group.
With regard to genotypic features, the analysis of agr subgroup did not reveal a significant association between a specific agr polymorphism and HVM or CC. We expected to find an association between a HVM and agrII polymorphism, relying on previous studies performed in MSSA bacteraemia [3,12]. In our study, agrII was two times more frequent in the HVM than in the LVM group (38% vs. 19%), but this did not reach statistical significance.
We did not identify a specific repertoire of virulence factors in the HVM group, as other previous studies did [6,7,10]. Although geographic regions are almost equally represented, changes in the genetic expression and phenotypic pattern in MSSA might be common over time, and geographic variations are also likely to occur as years pass. Another factor that could also have influenced the percentage of MSSA strains identified as HVM is the effect of freezer storage. Ludwig et al. [13] demonstrated a progressive decline of vancomycin MIC determination by Etest in methicillin-resistant Staphylococcus aureus bloodstream samples from the moment they were frozen.
This study has several limitations. First, the small sample size is a major shortcoming, greatly limiting the statistical power of the study and leading to the potential for a Type II error in the analysis. Second, the number of isolates tested and available is not representative of the overall ICE data set. However, we conducted a subanalysis comparing ICE left-sided MSSA frozen strains and those that were not frozen, and we did not detect significant differences regarding in-hospital and 1-year mortality and surgery rates (data not shown). Third, agrII dysfunction was not measured, so correlations with agr subgroup, vancomycin MIC and outcomes were not performed. Fourth, only cases occurring in the 2000–2006 period were included, which precluded observations regarding the temporal trends. Fifth, the potential variations of MSSA clones between geographic regions were not investigated. Finally, the specific type of β-lactam used was not available.
In conclusion, in this international cohort of left-sided MSSA IE treated with β-lactams, vancomycin MIC ≥1.5 μg/mL was not found to be an independent risk factor for complications of IE or for mortality. Stroke, paravalvular complications and some S. aureus genes were associated with a worse outcome. Differences in clonality and virulence factors were not found between strains with LVM and HVM. Further studies in this field are warranted to expand on these findings and to elucidate whether the contradictory results obtained in this field [14] are due to methodologic limitations or rather to a difficult-to-interpret phenomenon.
Supplementary Material
Acknowledgments
The ICE Investigators: Australia: E. Athan, O. Harris (Barwon Health), T. M. Korman (Monash Medical Centre), D. Kotsanas (Southern Health), P. Jones, P. Reinbott, S. Ryan (University of New South Wales). Brazil: C. Q. Fortes (Hospital Universitario Clementino Fraga Filho/UFRJ). Chile: P. Garcia, S. B. Jones (Hosp. Clínico Pont, Universidad Católica de Chile). Croatia: B. Barsic, S. Bukovski (Univ. Hospital for Infectious Diseases). France: C. Selton-Suty, N. Aissa, T. Doco-Lecompte (CHU Nancy-Brabois), F. Delahaye, F. Vandenesch (Hopital Louis Pradel), P. Tattevin (Pontchaillou University), B. Hoen, P. Plesiat, MD (University Medical Center of Besançon). Greece: H. Giamarellou, E. Giannitsioti, E. Tarpatzi (Attikon University General Hospital). Italy: E. Durante-Mangoni, D. Iossa, S. Orlando, M. P. Ursi, P. C. Pafundi, F. D’Amico, M. Bernardo, S. Cuccurullo, G. Dialetto, F. E. Covino, S. Manduca, A. Della Corte, M. De Feo (Università della Campania), M. F. Tripodi (University of Salerno). Lebanon: T. Baban, Z.A. Kanafani, S. S. Kanj, J. Sfeir, M. Yasmine (American University of Beirut Medical Center). New Zealand: A. Morris (Diagnostic Medlab), D. R. Murdoch (University of Otago). Slovenia: M. M. Premru, T. Lejko-Zupanc (Medical Center Ljubljana). Spain: M. Almela, J. Ambrosioni, M. Azqueta, M. Brunet, C. Cervera, E. De Lazzari, C. Falces, D. Fuster, C. Garcia-de-la-Mària, J. Garcia-Gonzalez, J.M. Gatell, M. Hernandez-Meneses, F. Marco, J. M. Miró, A. Moreno, J. Ortiz, S. Ninot, J. C. Paré, J. M. Pericas, E. Quintana, J. Ramirez, E. Sandoval, M. Sitges, J. M. Tolosana, B. Vidal, J. Vila (Hospital Clinic—IDIBAPS, University of Barcelona), E. Bouza, P. Muñoz, M. Rodríguez-Créixems, V. Ramallo (Hospital General Universitario Gregorio Marañón). United States: S. Bradley (Ann Arbor VA Medical Center), D. Wray, L. Steed, R. Cantey (Medical University of South Carolina), G. Peterson, A. Stancoven (UT-Southwestern Medical Center), C. Woods, G. R. Corey, L. B. Reller, V. G. Fowler Jr, V. H. Chu (Duke University Medical Center).
ICE Coordinating Centre: K. Baloch, V. H. Chu, G. R. Corey, C. C. Dixon, V. G. Fowler Jr, T. Harding, M. Jones-Richmond, P. Pappas, L. P. Park, T. Redick, J. Stafford.
ICE Publications Committee: K. Anstrom, E. Athan, A. S. Bayer, C. H. Cabell, V. H. Chu, G. R. Corey, V. G. Fowler Jr, B. Hoen, A. W. Karchmer, J. M. Miró, D. R. Murdoch, D. J. Sexton, A. Wang.
ICE Steering Committee: A. S. Bayer, C. H. Cabell, V. Chu, G. R. Corey, D. T. Durack, S. Eykyn, V. G. Fowler Jr, B. Hoen, J. M. Miró, P. Moreillon, L. Olaison, D. Raoult, E. Rubinstein, D. J. Sexton.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.cmi.2017.01.017.
Footnotes
Presented in part as abstract 2423 at the 55th Interscience Conference on Antimicrobial Agents and Chemotherapy, 18–21 September 2015, San Diego, California, USA.
Transparency Declaration
Supported in part by R01-AI068804 and K24-AI093969 (National Institutes of Health (NIH), USA), and by ‘La Marató de TV3’ (20152610) Barcelona, Spain; Instituto de Salud Carlos III and the Ministerio de Economia and Competitividad (FIS PI14/00603), Madrid, Spain; and a 2016 European Society of Clinical Microbiology and Infectious Diseases (ESCMID) research grant. JMP received a ‘Rio Hortega’ research grant (CM14/00135, 2015–16) from Instituto de Salud Carlos III and the Ministerio de Economia and Competitividad, Madrid, Spain, and a ESCMID/FEMS Joint Research Fellowship Award (2016). Instituto de Salud Carlos III, Ministerio de Economía y Competitividad, Madrid, Spain, provided to JMM a personal intensification research grant (INT15/00168 during 2016). VGF reports the following potential conflicts of interest: chair of the Scientific Advisory Board for Merck V710; paid consultant for Pfizer, Novartis, Galderma, Novadigm, Durata, Debiopharm, Genentech, Achaogen, Affinium, Medicines Co., Cerexa, Tetraphase, Trius, MedImmune, Bayer, Theravance, Cubist, Basilea and Affinergy; grants pending from MedImmune, Actavis/ Forest/Cerexa, Pfizer, Merck/Cubist, Advanced Liquid Logics, Theravance, Novartis, Medical Surfaces, and Locus Biotechnology; royalties from UpToDate; personal fees for development or presentation of educational presentations (Green Cross, Cubist, Cerexa, Durata, Theravance), outside the submitted work; and patent pending related to sepsis diagnostics. JMM has received consulting honoraria and/or research grants from AbbVie, Bristol-Myers Squibb, Cubist, Novartis, Gilead Sciences and ViiV. The other authors report no conflicts of interest relevant to this article.
References
- 1.Holmes NE, Turnidge JD, Munckhof WJ, Robinson JO, Korman TM, O’Sullivan MV. Antibiotic choice may not explain poorer outcomes in patients with Staphylococcus aureus bacteremia and high vancomycin minimum inhibitory concentrations. J Infect Dis. 2011;204:340–7. doi: 10.1093/infdis/jir270. [DOI] [PubMed] [Google Scholar]
- 2.Aguado JM, San-Juan R, Lalueza A, Sanz F, Rodríguez-Otero J, Gómez-Gonzalez C, et al. High vancomycin MIC and complicated methicillin-susceptible Staphylococcus aureus bacteremia. Emerg Infect Dis. 2011;17:1099–102. doi: 10.3201/eid1706.101037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viedma E, Sanz F, Orellana MA, San Juan R, Aguado JM, Otero JR, et al. Relationship between agr dysfunction and reduced vancomycin susceptibility in methicillin-susceptible Staphylococcus aureus causing bacteraemia. J Antimicrob Chemother. 2014;69:51–8. doi: 10.1093/jac/dkt337. [DOI] [PubMed] [Google Scholar]
- 4.López-Cortés LE, Velasco C, Retamar P, del Toro MD, Gálvez-Acebal J, de Cueto M, et al. Is reduced vancomycin susceptibility a factor associated with poor prognosis in MSSA bacteraemia? J Antimicrob Chemother. 2015;70:2652–60. doi: 10.1093/jac/dkv133. [DOI] [PubMed] [Google Scholar]
- 5.Cervera C, Castañeda X, de la Maria CG, del Rio A, Moreno A, Soy D, et al. Effect of vancomycin minimal inhibitory concentration on the outcome of methicillin-susceptible Staphylococcus aureus endocarditis. Clin Infect Dis. 2014;58:1668–75. doi: 10.1093/cid/ciu183. [DOI] [PubMed] [Google Scholar]
- 6.Nienaber JJ, Sharma Kuinkel BK, Clarke-Pearson M, Lamlertthon S, Park L, Rude TH, et al. Methicillin-susceptible Staphylococcus aureus endocarditis isolates are associated with clonal complex 30 genotype and a distinct repertoire of enterotoxins and adhesins. J Infect Dis. 2011;204:704–13. doi: 10.1093/infdis/jir389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bae IG, Federspiel JJ, Miro JM, Woods CW, Park L, Rybak MJ, et al. Heterogeneous vancomycin intermediate susceptibility phenotype in bloodstream methicillin-resistant Staphylococcus aureus isolates from an international cohort of patients with infective endocarditis: prevalence, genotype, and clinical significance. J Infect Dis. 2009;200:1355–66. doi: 10.1086/606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG, Jr, Ryan T, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–8. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 9.Fowler VG, Jr, Miro JM, Hoen B, Cabell CH, Abrutyn E, Rubinstein E, et al. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA. 2005;293:3012–21. doi: 10.1001/jama.293.24.3012. [DOI] [PubMed] [Google Scholar]
- 10.Fowler VG, Jr, Nelson CL, McIntyre LM, Kreiswirth BN, Monk A, Archer GL, et al. Potential associations between hematogenous complications and bacterial genotype in Staphylococcus aureus infection. J Infect Dis. 2007;196:738–47. doi: 10.1086/520088. [DOI] [PubMed] [Google Scholar]
- 11.Kalil AC, Van Schooneveld TC, Fey PD, Rupp ME. Association between vancomycin minimum inhibitory concentration and mortality among patients with Staphylococcus aureus bloodstream infections: a systematic review and meta-analysis. JAMA. 2014;312:1552–64. doi: 10.1001/jama.2014.6364. [DOI] [PubMed] [Google Scholar]
- 12.Holmes NE, Turnidge JD, Munckhof WJ, Robinson JO, Korman TM, O’Sullivan MV, et al. Genetic and molecular predictors of high vancomycin MIC in Staphylococcus aureus bacteremia isolates. J Clin Microbiol. 2014;52:3384–93. doi: 10.1128/JCM.01320-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ludwig F, Edwards B, Lawes T, Gould IM. Effects of storage on vancomycin and daptomycin MIC in susceptible blood isolates of methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 2012;50:3383–7. doi: 10.1128/JCM.01158-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Argudín MA, Mendoza MC, Méndez FJ, Martín MC, Guerra B, Rodicio MR. Clonal complexes and diversity of exotoxin gene profiles in methicillin-resistant and methicillin-susceptible Staphylococcus aureus isolates from patients in a Spanish hospital. J Clin Microbiol. 2009;47:2097–105. doi: 10.1128/JCM.01486-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.