Figure 2.
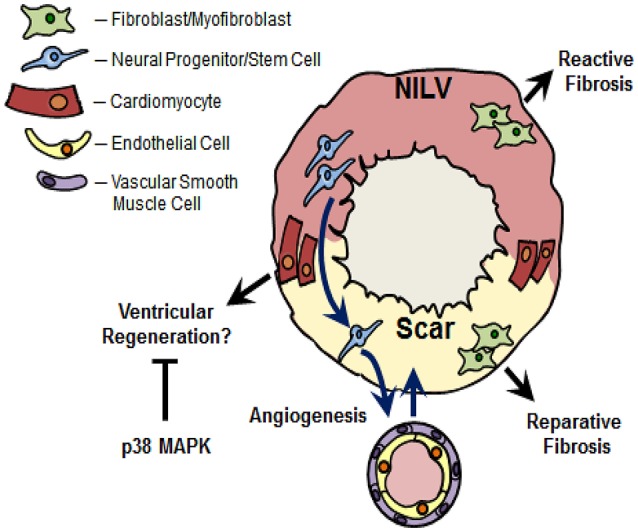
Nestin(+)-cells and cardiac remodeling following myocardial infarction. Ischemic injury to the adult rodent heart leads to the migration of cardiac resident nestin(+)-neural progenitor/stem cells from the non-infarcted left ventricle (NILV) to the scar region and subsequent differentiation to a vascular cell leading to de novo blood vessel formation. A subpopulation of cardiac resident nestin(+)-neural progenitor/stem cells also participate in the neurogenic response during scar formation (not depicted in figure). Recapitulation of the intermediate filament protein in scar-residing myofibroblasts may represent an activated phenotype to rapidly heal the infarct region during reparative fibrosis. The increased appearance of nestin(+)-fibroblasts was also reported in the fibrotic heart secondary to pressure-overload, in fibrotic lungs secondary to hypobaric hypoxia and the fibrotic kidney following unilateral ureteral obstruction. In this regard, the increased denisty of nestin(+)-fibroblasts may play a seminal role driving the reactive fibrotic response, regardless the tissue. Lastly, nestin was re-expressed in pre-existing adult cardiomyocytes detected predominantly at the peri-infarct/infarct region of the ischemically damaged heart. In vitro data revealed that nestin expression drives the cell cycle re-entry of neonatal rat ventricular cardiomyocytes. Collectively these data suggest that the appearance of nestin(+)-cardiomyocytes in the adult mammalian infarcted heart may represent an inherent paradigm of ventricular regeneration. Previous studies have reported that p38 MAPK inhibits the cell cycle re-entry and subsequent cytokinesis of ventricular cardiomyocytes. Based on the latter data, the modest appearance of nestin(+)-cardiomyocytes and concomitant inhibition of cell cycle re-entry may be likewise attributed in part to a suppressive action of p38 MAPK recruited by the overt inflammatory response post-myocardial infarction.
