Abstract
Heavy atom nanoparticles have high X-ray absorption capacity and near infrared (NIR) photothermal conversion efficiency, which could be used as radio-sensitizers. We hypothesized that concave PtCu octopod nanoframes (OPCNs) would be an efficient nanoplatform for synergistic radio-photothermal tumor ablation.
Methods: In this study, we newly exploited a folic acid-receptor (FR) mediated photothermal radiotherapy nanoagent base on OPCNs. OPCNs were synthesized with a hydrothermal method and then modified with polyethylene glycol (PEG) and folic acid (FA). A series of physical and chemical characterizations, cytotoxicity, targeting potential, endocytosis mechanism, biodistribution, systematic toxicological evaluation, pharmacokinetics, applications of OPCNs-PEG-FA for in vitro and in vivo infrared thermal imaging (ITI)/photoacoustic imaging (PAI) dual-modal imaging and synergistic photothermal radiotherapy against tumor were carried out.
Results: The OPCNs-PEG-FA demonstrated good biocompatibility, strong NIR absorption and X-ray radio-sensitization, which enabling it to track and visualize tumor in vivo via ITI/PAI dual-modal imaging. Moreover, the as-synthesized OPCNs-PEG-FA exhibited remarkable photothermal therapy (PTT) and radiotherapy (RT) synergistic tumor inhibition when treated with NIR laser and X-ray.
Conclusion: A novel multifunctional theranostic nanoplatform based on OPCNs was designed and developed for dual-modal image-guided synergistic tumor photothermal radiotherapy.
Keywords: PtCu nanoframe, targeting, near-infrared light, dual-modal imaging, synergistic tumor therapy.
Introduction
Although the progresses of anti-tumor therapeutics in the past few decades have greatly improved the quality of healthcare, malignant tumors are still the leading killers worldwide. In 2012 alone malignant tumors have caused 8.2 million deaths around the globe, and the morbidity rate is still on a rise each year 1, 2. Radiotherapy (RT), also called radiation therapy, is a widely used anticancer modality in clinic 3, 4. Typically, conventional radiotherapy was delivered via ionizing radiation like X-ray, which can induce effective necrosis of tumor tissue by directly damaging DNA and generation of reactive oxygen species 5. However, one consideration with radiotherapy is that it may also destroy those healthy cells and cause acute side effects such as nausea and vomiting, skin sores, hair loss, acute radiation syndrome (ARS), etc. 6. Another problem of radiotherapy is that the X-ray absorbency by solid tumors is usually unsatisfactorily low; therefore, patients have to receive a radiation dose which is actually much higher than that required for effective tumor suppression and thus inevitably cause severe injury to normal tissues. Moreover, reiterative radiotherapy could stimulate the mutation of cancer cells, resulting in the development of therapy resistance 7. To overcome the deficiency of radiotherapy, a series of radio-sensitizers, usually heavy atom nanoparticles (Z > 60), like gold (Au) 8, 9, bismuth (Bi) 10, 11, rare earth 12 and platinum (Pt) 13-15 have been greatly developed, which have high X-ray absorption capacity and photoelectric effect than that of soft tissues (average Z = 7.4). Due to the advantages, once irradiated with X-ray, various physical processes would occur such as photoelectric effect and Compton scattering. These effects allow the enhancing of the therapeutic effect at a relative lower and safer radiation dose. Therefore, radio-sensitizers have become to be promising nanoagents for tumor radiotherapy 16, 17.
Photothermal therapy (PTT) with NIR laser (700-1100 nm) has been proved an efficient approach, due to its minimal side effects on normal tissues and deep tissue penetration 18, 19. Various NIR-dependent photothermal nanomaterials were exploited as PTT agents, such as Au-based nanostructures 20, 21, carbon-based nanocomposites 22, and copper chalcogenide-based semiconductors, etc. 23. The photothermal conversion efficiency of these materials is tunable by controlling their elements, shapes, sizes, or aspect ratios. The Pt-based nanocomposites demonstrated great potential for photothermal therapy owing to its strong absorbance in the NIR region 24, 25, which also potentialize ITI and PAI guided tumor ablation. Meanwhile, recent studies proved that photothermal therapeutics assisting photodynamic therapy (PDT) could induce anti-tumor immunological effect by producing tumor-associate antigens in situ from ablated tumor, which also extended the application of photothermal nanoagents 26, 27. In addition, RT works efficiently with no depth restriction and PTT could efficiently kill tumors with limited side effects. Thus, the combination of RT and PTT therapeutics would be a promising approach to eliminate tumors, presenting prominent advantages in enhancing therapy efficacy and/or reducing side effects other than a single therapy. Furthermore, as an active drug delivery vehicle in vivo, satisfactory performance of biocompatibility, blood circulation and targeting efficiency should also be considered reasonably 28.
Herein, we report a radio-sensitizer for combined radiotherapy and photothermal therapy against tumor using folic acid and PEG modified octopod platinum-copper alloy nanoframes (OPCNs-PEG-FA). This nanoagent demonstrates several advantages, including: (a) the heavy-atom effect of OPCNs has great potential for enhancing the radio-sensitivity; (b) the OPCNs exhibited good photo-thermal conversion efficiency under NIR-laser irradiation; (c) PEG modification of OPCNs could improve the dispersity and stability of the nanoparticles in blood and thus prolong blood circulation time after intravenous (i.v.) injection; and (d) after the sequential conjugation with FA, the nanoparticles could target tumor cells effectively. On the other hand, the illumination of NIR lasers could be further facilitated by clinical interventional therapeutic technique such as endoscope-based devices. Thus, the OPCNs-PEG-FA reported in this study could efficiently inhibit tumor growth by synergistic photothermal radiotherapy.
Experimental
Materials
Chemicals were bought and used without further purification. SH-PEG-NH2 (Mw≈5000) was bought from Jenkem Co., Ltd. Platinum acetylacetonate (Pt(acac)2, 99%) and copper acetylacetonate (Cu(acac)2, 97%) were obtained from BOC Sciences. Oleylamine (OAM, 80-90%), cetyltrimethyl ammonium bromide (CTAB, 99%), cetyltrimethyl ammonium chloride (CTAC, 99%) was purchased from Aladdin. Folic acid (FA, 97%) was provided by Alfa Aesar Co (Tianjin, China).
Materials fabrication
Synthesis of oleylamine-coated concave PtCu nanoframes (OPCNs)
PtCu octopod nanoframes were synthesized solvothermally 29, 30. Briefly, 41.4 mg Pt(acac)2 and 138.4 mg Cu(acac)2 were added into a teflon-lined stainless steel autoclave (50 mL) containing 10 mL oleylamine under magnetic stirring for 2 h (Mixture Ⅰ). Meanwhile, 700 mg CTAB was dissolved into 10 mL oleylamine (Mixture Ⅱ). Then, Mixture Ⅱ was transferred to autoclave containing Mixture Ⅰ solution and sealed for reaction at 170 °C for 48 h. After cooling to ambient temperature, the black product was dispersed in methanol and collected by centrifugation at 4000 rpm for 5 min and further re-dispersed in cyclohexane.
Fabrication of oleylamine-free OPCNs
In a typical experiment, the prepared oleylamine-coated OPCNs above were dispersed into 10.0 mL cyclohexane mixing with 10 mL of deionized water. The solution was adjusted to pH 4 by HCl solution (0.1 M). Then, the reaction continued for 3 h with magnetic stirring. During this process, the carboxylate on the oleylamine surface was protonated (conversion into oleic acid) 31. Then, oleylamine-free PtCu nanoframes were dispersed into water and collected by centrifugation after precipitation with acetone. The precipitate was purified similarly with acetone for 6 times. Finally, the obtained oleylamine-free OPCNs were dispersed into water.
Synthesis of OPCNs-PEG
To increase the stability of nanoparticles in solution, the PEGylation of oleylamine-free OPCNs was conducted in this study 32. Typically, SH-PEG-NH2 (3.0 mM) solution was added into OPCNs solution (1.0 mM) to at an SH-PEG-NH2/OPCN molar ratio of 1000:1. The reaction was kept at room temperature for 20 h. Finally, the OPCNs-PEG nanocomposites were collected by centrifugation (11,000 rpm, 10 min) to remove the excessive unreacted PEG, and then re-dispersed in PBS.
Synthesis of OPCNs-PEG-FA
By using cross-linking reagents EDC and NHS, folic acid was covalently immobilized onto amine-functionalized OPCNs-PEG 33. Typically, 0.2 nM FA was added into OPCNs-PEG solution (20.0 mL, 0.1 mM) and reacted at room temperature for 12 h under magnetic stirring. The resulting OPCNs-PEG-FA nanoparticles were collected by centrifugation (11,000 rpm, 10 min) to remove the unreacted EDC, NHS and FA. The centrifuged OPCNs-PEG-FA nanocomposites were dispersed in PBS.
Fabrication of OPCNs-PEG@FITC and OPCNs-PEG-FA@FITC
To visualize the endocytosis of nanoparticles, FITC was further coated onto OPCNs-PEG and OPCNs-PEG-FA 34. Typically, 50 μM of FITC was added into OPCNs-PEG or OPCNs-PEG-FA solution (10 mg/mL) and then stirred at room temperature for 12 h. The OPCNs-PEG@FITC and OPCNs-PEG-FA@FITC nanocomposite was then collected by centrifugation (11,000 rpm, 10 min) to remove the unreacted FITC. The purified OPCNs-PEG@FITC nanoparticles were stored in PBS for further use.
Materials characterization
The morphologies of the as-synthesized nanoparticles were characterized by scanning electron microscopy (SEM, FEINova-400, Philips, Netherlands) and transmission electron microscope (TEM, LIBRA 200-FEG, Zeiss, Germany). High angle annular dark field scanning transmission electron microscope (HAADF-STEM) images and EDS mappings were recorded by a Titan G2 80-200 electron microscopy with energy dispersive X-ray (EDX) detector system. The crystalline structure of PtCu nanoframes was recorded with an X-ray diffraction (D/max 2500-PC, Rigaku, Japan) with Cu Kα radiation (λ = 1.54 Å). The surface charges of particles were measured by a potentiometric analyser (BIC-ZetaPALS, Brookhaven, USA).
NIR absorption of OPCNs-PEG-FA
The UV-vis-NIR absorption of OPCNs-PEG-FA was measured in this study. OPCNs-PEG-FA (200 μg/mL) in PBS was irradiated with NIR laser (2.4 W/cm2, 5 min) at 778 nm, 808 nm and 980 nm, respectively. Then the UV-vis-NIR absorbance spectrum of OPCNs-PEG-FA was obtained by a UV-vis-NIR spectrophotometer (NanoDrop One, Thermo Fisher Scientific, USA).
Thermogenesis of OPCNs-PEG-FA
Thermogenesis property of the OPCNs-PEG-FA was evaluated under NIR laser irradiation. Typically, the OPCNs-PEG-FA nanoparticles with different concentrations (50, 100, 200 and 300 μg/mL) in PBS was irradiated with NIR laser (808 nm, 2.4 W/cm2, 5 min). To evaluate the photothermal generation stability of the as-synthesized nanoparticles, the OPCNs-PEG-FA (200 μg/mL) was re-exposed to 808 nm NIR laser for 5 irradiation cycles.
In vitro study
ITI/PAI imaging in vitro
To test the linearity of the in vitro ITI signal of OPCNs-PEG-FA, different concentrations of the nanoparticles (0, 50 and 200 μg/mL) in PBS were irradiated with NIR laser (808 nm, 2.4 W/cm2) for 5 min. The ITI images were recorded by an IR-thermal camera (P20, FLIR-Systems, USA). Meanwhile, to investigate the linearity of the PAI signal of OPCNs-PEG-FA, various concentrations of OPCNs-PEG-FA nanoparticles (0, 25, 50, 100, 150 and 200 μg/mL) were filled in 2.5% agarose gel tubes (1.0 mL) for in vitro PAI signal detection.
Cell culture
HepG2, KB and Hela cells were cultured with MEM medium containing 10% of fetal bovine serum (FBS) and 1% (v/v) of penicillin-streptomycin. A549 cells were cultured with F-12K medium containing 10% of FBS and 1% (v/v) of penicillin-streptomycin. C6 cells were cultured with DMEM medium containing 10% of FBS and 1% (v/v) of penicillin-streptomycin. All cells were incubated at 37 °C under 4.5% CO2 atmosphere.
Study of cellular uptake mechanism
To test whether the phagocytosis process of OPCNs-PEG-FA nanoparticles is energy-dependent or not, we used low temperature condition (4 °C), sodium azide (0.1%, v/v) and 50 mM deoxyglucose (NaN3/DOG) to inhibit ATP generation during the uptake of OPCNs-PEG-FA nanoparticles 35, 36. To study the endocytosis pathway of OPCNs-PEG-FA, HepG2 cells were pre-incubated with following inhibitors: sucrose, chlorpromazine, filipin, methyl-β-cyclodextrin (Mβ-CD), colchicines and wortmannin, respectively. Prior to the inhibition experiment, the toxicity of each endocytosis inhibitor was assessed by MTT assay. Briefly, cells were seeded into 96-well plates at a density of 1×104 cells/well and then incubated with following inhibitors: 150 mg/mL sucrose, 10 µg/mL chlorpromazine, 4 µg/mL filipin, 100 mg/mL Mβ-CD, 20 µg/mL wortmannin and 40 µg/mL colchicines at 37 °C 37, 38. After 48 h of incubation, cells were washed with PBS and then 100 µL MTT (5 mg/mL) was added to each well, and the plates were further incubated for 4 h. The medium in each well was removed and 100 µL DMSO was added to dissolve the formed formazan crystals. Cell viability was measured with a microplate reader (Bio Rad-680, USA) at a wavelength of 570 nm. After pre-treatments HepG2 cells with above inhibitors as the same concentrations for 6 h, OPCNs-PEG-FA@FITC nanoparticles (200 µg/mL) were added and incubated for another 1 h, and the quantitative uptake of OPCNs-PEG-FA@FITC was then determined by fluorescent assay.
In vitro cytotoxicity assay
The cytotoxicity of OPCNs-PEG-FA was assessed with MTT assay. Briefly, HepG2 cells were seeded in 96-well plates with a cell density of 7.0 × 103 cells/cm2. When cell confluence reached around 80%, culture medium containing OPCNs-PEG-FA with different concentrations (25, 50, 100, 200 μg/mL) was added and irradiated with NIR laser (808 nm, 2.4 W/cm2, 5 min) and X-ray (120 kvp, 10 Gy, 10 min) individually or in conjunction. After treatments for 12 h, cell viability was determined with MTT assay as above.
In vivo study
Animal model
All animal experiments were strictly complied with the guideline of the Institutional Animal Care and Use Committee of China. 4-week female nude mice (average weight ~ 25 g) were bought from animal experimental centre of Xinqiao Hospital (Chongqing). The mice were subcutaneously injected with 100 μL cell suspension containing 2 × 106 HepG2 cells to develop tumors.
In vivo synergistic photothermal radiotherapy studies
To investigate the synergistic tumor suppression of photothermal/radiotherapy, the tumor-bearing mice were randomly divided into 5 groups (five mice per group): control (PBS), radiotherapy, nanoparticle-enhanced radiotherapy (OPCNs-PEG-FA+RT), photothermal therapy (OPCNs-PEG-FA+PTT) and photothermal radiotherapy (OPCNs-PEG-FA+RT+PTT). When tumor volumes reached to about 60 mm3, the nanoparticles (2.0 mg/mL, 100 µL) administrated mice were irradiated with NIR laser (808 nm, 2.4 W/cm2, 5 min) and X-ray (120 kvp, 10 Gy, 10 min) alone or together. The volume of tumor was calculated with the formula: Vtumor = length × width2/2. The relative volume was defined as (V/V0 (V: current volume, V0: initial tumor volume).
In vivo multimodal imaging
After i.v. injection of OPCNs-PEG-FA (50, 100 and 200 μg/mL, 100 µL) and irradiated with NIR laser (808 nm, 2.4 W/cm2, 5 min), then the infrared thermal images were recorded with an IR-thermal camera. The PAI images were measured with a photoacoustic 3D tomographic imaging system (Veco®LAZR, VisualSonics, Canada) at 1, 2 and 6 h.
In vivo pharmacokinetics
Mice were administrated with OPCNs-PEG-FA (4.0 mg/kg) via tail vein injection. At different time points (0, 0.5, 1, 1.5, 2, 4, 6, 12, 24, 48 h), 50 μL whole blood were collected through eyeball extirpating. Cu content in each sample was recorded by ICP-AES. The pharmacokinetic parameters were calculated with the model as reported 39.
In vivo biodistribution studies
After i.v. injection of OPCNs-PEG-FA, mice were ultimately killed at 1 and 48 h. Heart, liver, spleen, lung, kidney, skin, muscle, bone, stomach and tumor from each mouse were collected after tissue homogenation and manually grounding and transferred into test tubes. Then the Cu content in main organs was detected by ICP-AES.
In vivo systematic toxicological evaluation
To investigate the hemocompatibility of OPCNs, OPCNs-PEG and OPCNs-PEG-FA, hemolysis ratio (HR) and coagulation time were recorded. In this study, a hemolysis ratio (HR) test was taken to detect hemoglobin release in vitro. Typically, five healthy mice were intravenously injected with OPCNs-PEG-FA (100 mg/kg-1), while saline was used as control. At the 1st, 14th, and 28th day post i.v. injection, the blood samples from mice were collected for blood biochemical test and hematology analysis. Meanwhile, the tumors and main organs including heart, liver, lung, spleen and kidney were fetched for staining with haematoxylin-eosin (H&E). To evaluate the apoptosis in vivo, tumor tissue was stained with TUNEL apoptosis kit.
Statistical analysis
All data were presented as mean with standard deviation (SD). Statistical analysis was analysed with OriginPro (version 8.0) via one-way analysis of variance (ANOVA) and Students's t-test. The confidence levels were set as 95% and 99%.
Results and Discussion
Materials synthesis and characterization
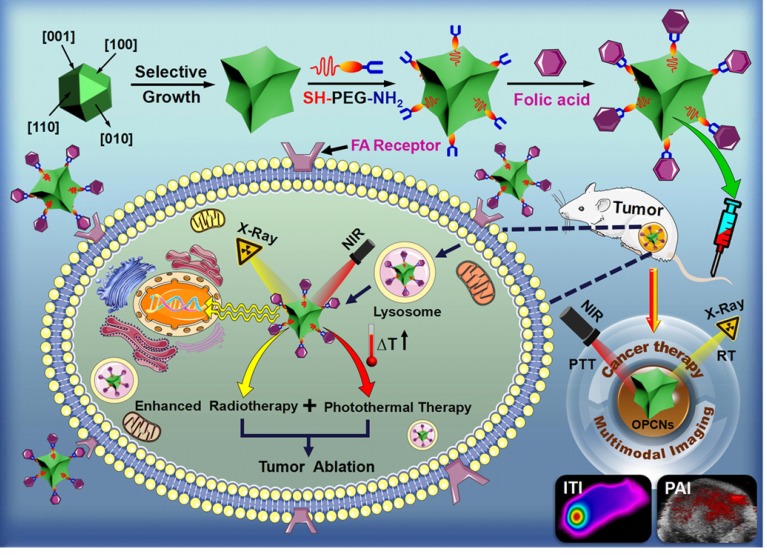
The Scheme 1 shows the preparation process of OPCNs-PEG-FA and its actions against tumor cells. PEG coating of OPCNs would extend its circulation time after i.v. injection and FA conjugation would facilitate the internalization of OPCNs-PEG-FA by tumor cells through FA receptor recognition. Afterwards, by irradiating with X-ray and NIR laser, the endocytosed OPCNs-PEG-FA would lead to heat production via photothermal conversion and enhanced radiotherapy, resulting in the synergistic killing of tumor cells and tumor growth inhibition. Meanwhile, ITI and PAI imaging could facilitate the localization of the tumor and monitor its curative effect.
Scheme 1.
Schematic preparation of OPCNs-PEG-FA and its synergistic photothermal radiotherapy against tumor cells with X-ray and NIR laser irradiation.
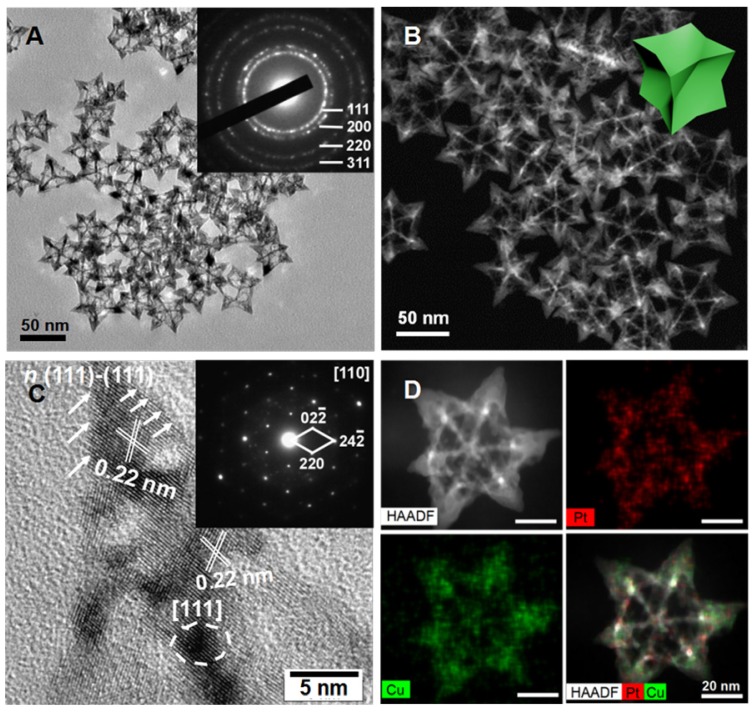
In this study, octopod platinum-copper nanoframes (OPCNs) were synthesized via a solvethermal method. The morphology and crystallization during nanoparticle preparation were recorded at 1, 6 and 48 h (Fig. S1). The nanoparticles displayed a time-dependent growth behavior. Then, the ultimate morphologies of the as-synthesized nanoframes were characterized with multiple microscopic techniques. As shown in Fig. 1 A & B and Fig. S2, the TEM, HAADF-STEM and SEM images show that the obtained products are mono-dispersive nanocrystals with uniform morphology. The well-defined nanostructure possesses narrow size distribution with an average size of about 64.2 nm. Interestingly, angle-dependent HAADF-STEM images indicate that the OPCNs had a concave structure (Fig. S3), which presenting eight symmetric feet with average edge breadth of about 42.6 nm and feet length of around 20.7 nm (Fig. S4). Moreover, Fig. 1 C indicates the high-resolution TEM image of an individual foot oriented along the [110] zone axis, where numerous step and terrace atoms could be found on the surfaces of the OPCNs nanoframes. The HAADF-STEM images of the octopod nanoframes clearly demonstrate that Pt and Cu elements were homogeneously distributed throughout the Pt-Cu alloy nanoframes (Fig. 1 D).
Figure 1.
(A) TEM image of the PtCu OPCNs, the inset image shows the diffraction rings of individual CONFs; (B) The HAADF-STEM image of the as-synthesized OPCNs; (C) High resolution TEM image of one foot on the OPCNs, the white arrows show the high-index facets of n (111)-(111); The inset image shows the corresponding fast Fourier transition pattern; and (D) HAADF-STEM and EDX mapping images of an individual OPCNs.
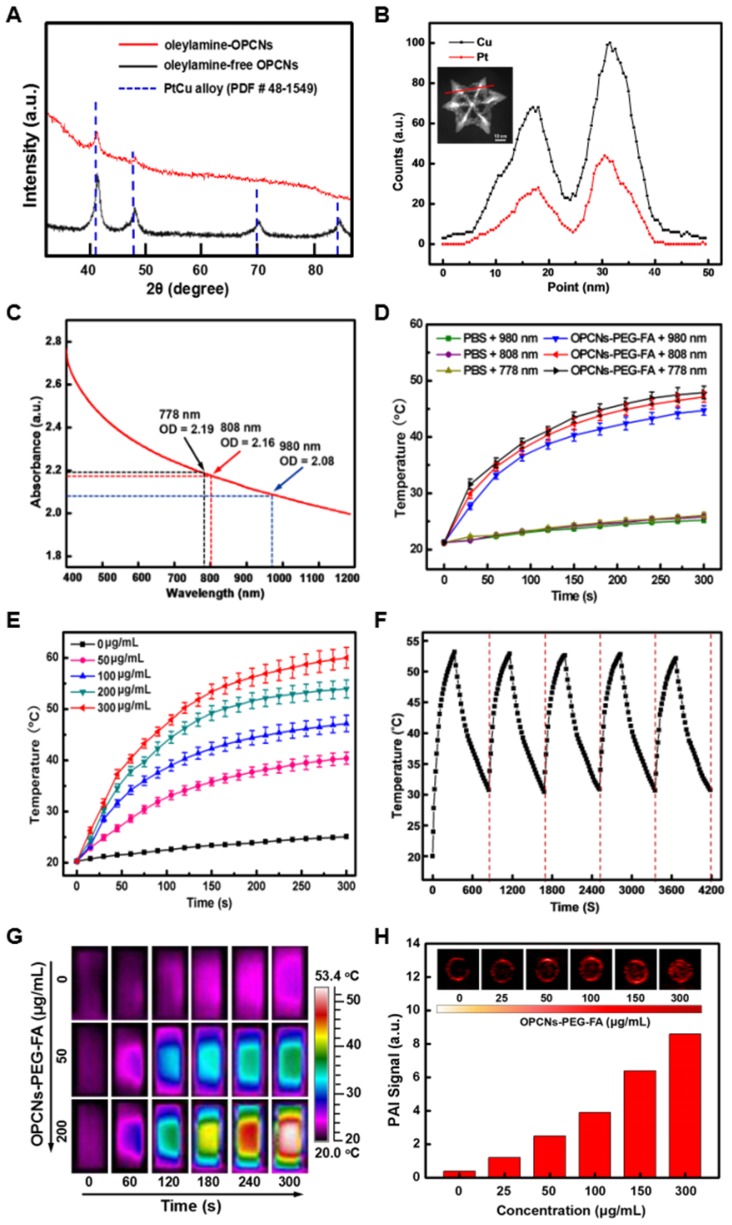
Meanwhile, to investigate the composition and crystal structure of the OPCNs, XRD analysis was carried out for further characterization. As shown in Fig. 2 A, the XRD patterns of the oleylamine-capped and oleylamine-free OPCNs exhibit a typical face-centered-cubic (FCC) and single-phase Pt-Cu alloy structure, the typical peaks located at 2θ = 40.15, 46.56, 68.18, and 82.23° were assigned to (111), (200), (220), and (311) planes 40. The diffraction peaks of OPCNs shift to larger angle (about 0.5 degree) compared with the PtCu alloy (PDF No: 48-1549), indicating the high Cu content in OPCNs. The element analysis by energy-dispersive X-ray (EDX) spectrometry further demonstrates that the mass ratio of Pt: Cu in OPCN was about 1: 2.5 (Fig. 2 B). The composition of the obtained OPCNs was also supported by the XPS results. As shown in Fig. S5 a, the XPS spectra of OPCNs reveal the presence of Pt and Cu 41. Two peaks at 71.2 and 74.5 eV for the OPCNs (Fig. S5 b) were attributed to the Pt 4f7/2 and Pt 4f5/2. The peaks at 932.5 and 953.1 eV were assigned to the binding energies of the 2p3/2 and 2p1/2 electrons of Cu (Fig. S5 c).
Figure 2.
Characterization of OPCNs nanoparticles: (A) X-ray diffraction patterns of oleylamine-coated OPCNs and oleylamine-free OPCNs, respectively; (B) EDX line-scanning profiles of an individual OPCNs; (C) The UV-Vis absorbance spectrum of OPCNs-PEG-FA in the NIR region; (D) Thermogenesis capacity of OPCNs-PEG-FA irradiated by NIR laser with different wave length; (E) The concentration-dependent temperature change of OPCNs-PEG-FA in PBS irradiated by NIR laser (808 nm, 2.4 W/cm2, 5 min); (F) Temperature change of the OPCNs-PEG-FA (200 μg/mL) in PBS over 5 On/Off cycles of NIR laser irradiation; (G) Infrared thermographic images of OPCNs-PEG-FA irradiated with NIR laser; and (H) Concentration-dependent photoacoustic images of OPCNs-PEG-FA irradiated with 808 nm NIR laser in vitro.
After the functionalization with PEG and FA, the surface properties of the nanoparticles would be changed. To this end, FTIR spectroscopy was further employed to characterize the synthetic process (Fig. S6). Typically, the characteristic peaks of OPCNs were observed at 502.52 cm-1, which was the plasma resonance of PtCu alloy nanoframes. For SH-PEG-NH2 sample, peak at 2880.81 cm-1 was attribute to the symmetrical stretching vibration of -CH2-; the peaks at 1376.77 cm-1, 1244.75 cm-1, 1114.89 cm-1, 954.11 cm-1 and 849.93 cm-1 matched the characteristic peaks of PEG, and the characteristic absorption band of -SH was observed at 2555.40 cm-1; while the peaks at 1636.36 cm-1 and 1466.93 cm-1 were assigned to the stretching vibration of -NH 42. After surface PEGylation with SH-PEG-NH2, no absorption band of -SH was observed in the OPCNs-PEG sample, while the characteristic absorption bands of -NH2 and PEG remained, which was attributed to the reaction between thiol groups from OPCNs nanoparticles (Pt-SH) and SH-PEG-NH2 molecules 43. For pure FA sample, peak at 1606.51 cm-1 was assigned to stretching vibration of benzene, peaks at 1571.19 cm-1 and 1412.88 cm-1 were attributed to abundant -COOH groups. Finally, FA immobilized OPCNs-PEG displayed a new peak at 1607.37 cm-1 due to the characteristic benzene vibration bonds of FA, the peaks at 1118.24 cm-1 and 840.77 cm-1 derived from the characteristic stretch bands of PEG, respectively. Additionally, the appearance of 1650.52 cm-1 peak was the characteristic amide bonds I and II due to the condensation (PEG-CONH-FA) with amino groups in PEG-NH2 tail and -COOH groups in FA 44. All results of FTIR spectra demonstrated that the PEG coating and FA conjugation occurred. Meanwhile, the zeta potential measurements also proved that the desired nanoparticles were successfully fabricated (Fig. S7).
To evaluate the photothermal capacity of OPCNs-PEG-FA, the NIR light absorbance and temperature change of OPCNs-PEG-FA solution under NIR laser irradiation were monitored. The UV-Vis spectra show that the nanoagents had relatively high absorbance in near infrared region (Fig. 2 C), indicating its great potential for NIR-dependent PTT. Moreover, to investigate the thermogenesis of the nanoparticles for tumor therapy, different concentrations of nanocomposites were irradiated by 808 nm laser for 5 min, the temperature rapidly reached to above 53 °C with nanoparticles concentration of 200 μg/mL (Fig. 2 D & E). These results reveal that the nanoparticles could be potentially adopted as PTT agents for tumor thermal ablation. Moreover, the photothermal stability of OPCNs-PEG-FA was detected by the treatment of 808 nm NIR light for 5 on-off cycles (Fig. 2 F). No obvious decrease of thermogenesis occurred after 5 cycles, indicating that the nanoagents had quite good photothermal stability. In addition, the nanoagent was dispersed into PBS, saline and cell medium for evaluating its stability. The result suggests that OPCNs-PEG-FA nanoparticles have good stability in all solutions (Fig. S8 a). Moreover, the thermogenic capacity of the nanoagent exhibits no significant difference in different solutions (Fig. S8 b).
To evaluate the feasibility of the nanoagent for therapeutic imaging application, calorigenic imaging of OPCNs-PEG-FA (200 μg/mL) was recorded by irradiating with NIR laser. The infrared thermographic images demonstrate that OPCNs-PEG-FA had good thermogenesis (above 50 °C) under 808 NIR laser for 5 min (Fig. 2 G). On the other hand, PAI, a non-invasive imaging modality, presents excellent high tracer depth and spatial resolution than those conventional optical imaging methods. To this end, we study the possibility whether the OPCNs-PEG-FA could be served as an ideal PAI contrast. In our study, photoacoustic signal corresponding increases with the concentration from 25 to 200 µg/mL (Fig. 2 H), indicating the OPCNs-PEG-FA would be a good candidate for ITI and PAI diagnosis.
In vitro evaluations
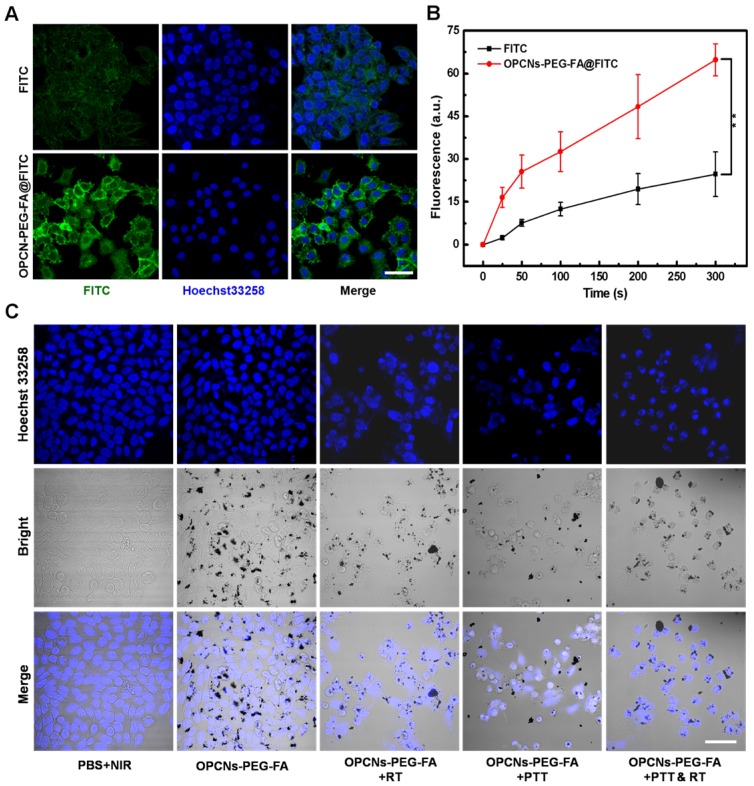
Before the dual-model therapy nanoagent adopted for tumor therapy application, we firstly measured the endocytosis and cytotoxicity of the OPCNs-PEG-FA in vitro. To investigate the interaction and distribution of OPCNs-PEG-FA in HepG2 cells, we employed confocal laser scanning microscopy (CLSM) to observe cell morphology in different groups 45. With the help of FA, the phagocytosis processes of OPCNs-PEG-FA@FITC by HepG2 cells were evidently accelerated under CLSM (Fig. 3 A, B).
Figure 3.
(A) CLSM images of HepG2 cells incubated with FITC alone and OPCNs-PEG-FA@FITC (200 μg/mL) nanoparticles for 6 h; (B) Fluorescence signal of OPCNs-PEG-FA@FITC in HepG2 cells (n = 5); and (C) CLSM images of HepG2 cells incubated with OPCNs-PEG-FA under different conditions. Scale bar: 50 μm.
The targeting capacity of OPCNs-PEG-FA mediated by folic acid-receptor (FR) was evaluated in both FR positive (FR+) and negative (FR‒) tumor cells by flow cytometry (FCM) assay, including KB (FR+++), Hela (FR++), C6 (FR+), HepG2 (FR‒) and A549 (FR‒ ‒) cells 46. As shown in Fig. S9 a, the phagocytosis efficiency follows the order of KB>Hela>C6>HepG2>A549. Moreover, the FR-mediated cell uptake was attenuated by prior addition of free folic acid, which suggests that the OPCNs-PEG-FA could be used as an excellent candidate for FR-mediated targeted drug delivery. Meanwhile, the study also reveals that the cellular uptake of OPCNs-PEG-FA nanoagent was a dose- (Fig. S9 b) or time-dependent process (Fig. S9 c).
Generally, transport of nanoparticles across cells membranes is energy consuming 47. To this end, inhibitors were used to inhibit ATP generation during cell uptake of nanoparticles. Cells were treated at low temperature (4 °C) or with NaN3/DOG to determine whether the endocytosis pathway was energy-dependent or not. As shown in Fig. S10 a, the cell uptake efficiency significantly decreased either by treatment at 4 °C or with NaN3/DOG, which suggests that cell uptake of OPCNs-PEG-FA was an energy-dependent process.
Moreover, the cell uptake of nanocarriers could be realized via multiple pathways, mainly including clathrin-mediated endocytosis, caveolae-mediated endocytosis, and macropinocytosis 48, 49. To reveal the potential phagocytosis pathway of OPCNs-PEG-FA, the inhibition of clathrin-mediated uptake was firstly investigated by using inhibitors of sucrose and chlorpromazine. The mean fluorescence intensity decreased to 61.7% for sucrose and 72.4% for chlorpromazine, respectively. The results indicate that clathrin-mediated endocytosis was involved in the cellular uptake of OPCNs-PEG-FA nanoparticles. Subsequently, filipin (inhibitor for caveolae-mediated endocytosis) was used to evaluate the caveolae-mediated uptake. Compared to control group, the mean fluorescence intensity decreased to 56.1%. The result suggests that caveolae-mediated pathway was the main approach for the internalization of OPCNs-PEG-FA. Moreover, Mβ-CD (inhibitor for both clathrin-mediated and caveolae-mediated endocytosis) was adopted to evaluate the cell uptake, the mean fluorescence intensity decreased to 29.1%. The results reveal that both clathrin-mediated and caveolae-mediated endocytosis pathways participated in the cell entry of OPCNs-PEG-FA. In addition, to investigate whether macropinocytosis-mediated endocytosis was involved in the cellular uptake of OPCNs-PEG-FA, wortmannin (phosphatidyl inositol-3-phosphate inhibitor) and colchicine (microtubule-disrupting agent) were used in our study. The results suggest that these two inhibitors had little inhibitory effect on cell uptake of OPCNs-PEG-FA (Fig. S10 a). Furthermore, the six inhibitors were proved to have relatively low cytotoxicity against HepG2 cells (Fig. S10 b). In short, these findings indicate that the cell uptake of OPCNs-PEG-FA was an energy-dependent process through clathrin /caveolae mediated endocytosis.
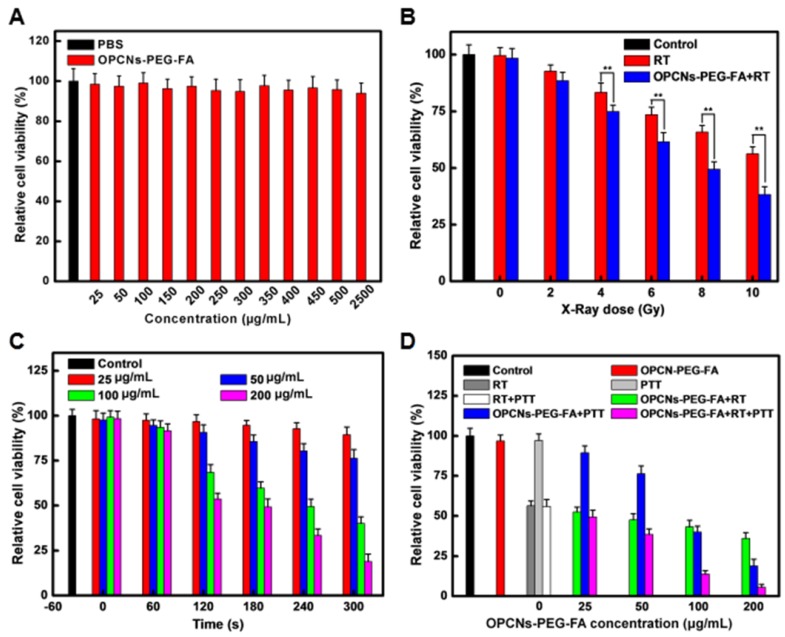
We next employed CLSM to record the cell morphology under RT and NIR laser irradiation, as shown in Fig. 3 C, the photothermal radiotherapy led to significant cell apoptosis. Meanwhile, methyl thiazolyl tetrazolium (MTT) assay was employed to evaluate the relative cytotoxicity of OPCNs-PEG-FA in vitro. No remarkable cytotoxicity was detected even at a high concentration of 2500 μg/mL (Fig. 4 A), indicating that the nanoparticles had good biocompatibility. We further evaluated the feasibility of OPCNs-PEG-FA for combined RT and PTT against tumor cells. Typically, HepG2 cells were firstly incubated with OPCNs-PEG-FA (200 μg/mL) in PBS for 6 h and then treated with RT and PTT. As shown in Fig. 4 B & C, when HepG2 cells treated with RT or PTT (808 nm, 2.4 W/cm2) alone, the OPCNs-PEG-FA exhibits favourable radiotherapy sensitization in dose- or time-dependent manner. The radio-sensitization of OPCNs-PEG-FA nanoparticles was measured to determine their potential in radiotherapy 16. A colony formation assay of HepG2 cells irradiated by X-ray with different doses (0, 2, 4, 6, 8 and 10 Gy) was performed in presence or absence of OPCNs-PEG-FA. The results were fitted with a multitarget- single-hitting model using the following Equation 50:
Figure 4.
(A) Cell viabilities of HepG2 cells after incubation with various concentrations of OPCNs-PEG-FA nanoparticles for 24 h (n=5); (B) Cell viabilities of HepG2 cells exposed to different radiation dosages with the assistance of OPCNs-PEG-FA; (C) Relative viabilities of HepG2 cells irradiated by NIR laser with different concentrations of OPCNs-PEG-FA; and (D) Cell viabilities after the synergistic photothermal radiotherapy treatment with different concentrations of OPCNs-PEG-FA with defined X-ray dose (10 Gy).
| S = 1 ‒ [1 - exp(- D / D0)]^N | (1) |
Where S is the cell survival ratio after X-ray irradiation; D is the X-ray dose; D0 is the dose of X-ray when the survival ratio drops to 66.7% in the exponential curve, which means the average dose of X-ray for killing cells; N is an extrapolated value to characterize the cells' self-repairing capability against X-ray irradiation. The quasi-threshold dose (Dq) and sensitivity enhancement ratio (SER) could be calculated by Equations 2 & 3. The Dq and SER are the main indicators to reflect radio-sensitivity 51.
| Dq = In(N * D0) | (2) |
| SER = D0 (ctrl.) / D0 (exp.) | (3) |
It was found that HepG2 cells incubated with OPCNs-PEG-FA exhibited obvious decrease in survival rate under X-ray irradiation, when comparing with that of control. Moreover, the SER value of OPCNs-PEG-FA nanoparticles was estimated to be 1.57 (X-ray dose: 10 Gy), which was consistent with previous studies 52, 53.
When HepG2 cells were treated with RT and PTT combined, the cell viability dramatically dropped below 5%. It was about 40% lower than that of sole OPCNs-PEG-FA enhanced radiotherapy and 20% lower than that of sole OPCNs-PEG-FA mediated photothermal treatment (Fig. 4 D). Furthermore, quantitative study of the interaction between radiotherapy and photothermal therapy was performed in our study. Typically, combination index (CI) assay is a widely-used approach for evaluating synergistic effects of combination therapy. By employing the Loewe additivity model, the combined effects of two therapeutic regimens were calculated by the following Equation 54:
| (D)1/(Dx)1 + (D)2/(Dx)2 = 1 | (4) |
where (D)1 and (D)2 are the combination doses of enhanced RT and PTT that achieve an effect of 50% cell growth inhibition (GI 50); the (Dx)1 and (Dx)2 are the corresponding doses for single RT or PTT that resulting in the same effect, i.e., 50% growth inhibition of RT and PTT alone. When the Equation 4 holds, it reflects that the synergic effect of the two therapeutic methods are additive. Then the combination index could be calculated to determine if the interactions of RT and PTT is synergistic, additive or antagonistic by the deduced Equation 5:
| CI = (D)1/(Dx)1 + (D)2/(Dx)2 | (5) |
Where CI < 1 indicates synergy; CI = 1 means additivity; CI > 1 presents antagonism 55. We used this model to simulate the anti-proliferative effect and to calculate the CI value of combination enhanced RT + PTT by an isobologram analysis (Fig. S11). The computed CI value for the combination is about 0.746, suggesting that OPCNs-PEG-FA nanoparticles mediated enhanced RT + PTT synergistic therapy.
The synergistic tumor inhibition induced by OPCNs-PEG-FA was further demonstrated by flow cytometry analysis (Fig. S12). The results showed that DNA strands were severely damaged after X-ray irradiation (120 kvp, 10 Gy, 10 min) and NIR laser (808 nm, 2.4 W/cm2, 5 min) in the presence of OPCNs-PEG-FA (200 µg/mL). It could be found that the RT and PTT combination led to severe late stage apoptosis ultimately.
In vivo evaluations
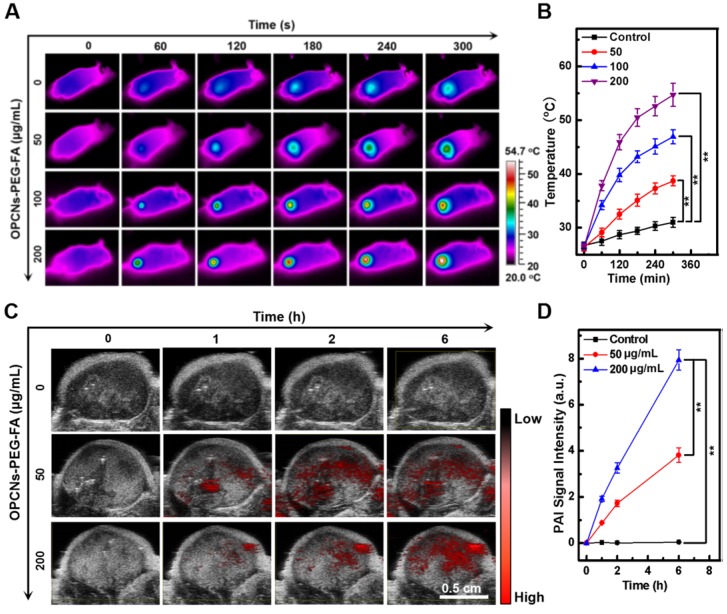
The multimodal imaging capacity of the OPCNs-PEG-FA was then evaluated in HepG2 tumor bearing nude mice. After i.v. injection of OPCNs-PEG-FA and irradiation with NIR laser, we firstly employed an IR thermal camera to monitor the temperature change (Fig. 5 A), which was recorded at specific time intervals. Accordingly, nude mice injected with OPCNs-PEG-FA presented obvious heat generation at tumor region in a concentration-dependent manner. As expected, under the 808 nm laser, the tumor surface temperature in OPCNs-PEG-FA group rapidly increased from 27.4 to 54.7 °C (Fig. 5 B), which was sufficient to ablate tumor cells; whereas the surrounding tissue near the tumor exhibited near physiological temperature. In contrast, temperature of nude mice injected with PBS showed no significant increase (from 27.6 to 35.1 °C) during NIR laser irradiation.
Figure 5.
Imaging-guided combined photothermal radiotherapy by OPCNs-PEG-FA in vivo. (A) Infrared thermographic images of tumor-bearing nude mice irradiated with NIR laser and (B) Temperature change during the combined chemo-photothermal therapy process (n=3); (C) PAT signals in the tumors before and after injection of OPCNs-PEG-FA for 0, 1, 2 and 6 h; and (D) PAT signal changes during the cancer therapy process (n=3).
Next, tumor bearing mice were intravenously injected with OPCNs-PEG-FA and observed with a photoacoustic computed tomography scanner, the PAI signal was recorded at 0, 1, 2, and 6 h (Fig. 5 C). The PAI signal of the OPCNs-PEG-FA remarkably increased over time (Fig. 5 D), indicating the effective tumor retention of OPCNs-PEG-FA through both passive enhanced permeability and retention (EPR) effect and active FA mediated targeting pathway. Meanwhile, the results also indicate that the OPCNs-PEG-FA nanoparticles could be used as an excellent PAI nanoagent for imaging of the tumor area and monitoring the curative process of tumor therapy.
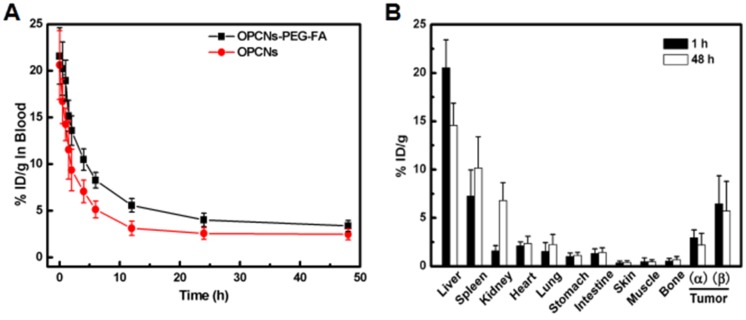
To investigate the efficacy of photothermal radiotherapy with OPCNs-PEG-FA nanoplatform for potential tumor inhibition, we performed detailed in vivo evaluations. A pharmacokinetic study was firstly carried out after i.v. injection. We detected the blood circulation behaviour of OPCNs-PEG-FA nanoparticles at different time intervals. As shown in Fig. 6 A, OPCNs-PEG-FA could be cleared out of blood stream within 12 h. A biodistribution evaluation of OPCNs-PEG-FA was also conducted to assess the targeting efficiency via EPR effect and FA targeting in vivo. Typically, tumor bearing mice were intravenously injected with OPCNs-PEG-FA. At 1 h and 48 h after injection, mice were euthanatized and subjected to heart perfusion to minimize the influence from intrinsic Cu storage in blood when detecting Cu content in tissue. Subsequently, organs and tumors were dissected for Cu content measurement. The results show that higher OPCNs-PEG-FA (β) accumulation in tumor was achieved than that of bare OPCNs (α) both at 1 and 48 h after injection (Fig. 6 B). The result reveals that OPCNs-PEG-FA could be readily internalized by tumor cells after PEGylation and FA modification. At both time points, mice treated with OPCNs-PEG-FA have high Cu accumulation in liver. The biodistribution of the nanoparticles in kidney was 1.78% at 1 h and 4.79% at 48 h respectively, indicating the bio-clearance of nanoparticles through renal metabolism potentially 56.
Figure 6.
(A) Blood retentions of OPCNs-PEG-FA in HepG2 tumor bearing mice during 48 h after intravenously injections; and (B) Biodistribution of OPCNs-PEG-FA in the major organs at 1 and 48 h post injections (n = 5), where α means OPCNs and β means OPCNs-PEG-FA.
Furthermore, it was noted that the OPCNs-PEG-FA concentration in the blood was time-dependent, in which two distinct regions have been found especially relevant to its clearance in vivo. The first region (0~12 h) indicates the initial clearance of OPCNs-PEG-FA from circulation, and the second region (12 ~ 48 h) shows the terminal clearance of OPCNs-PEG-FA nanoparticles. The initial region implies the OPCNs-PEG-FA nanoparticles distribution in vascular and extravascular tissues, while the posterior half-life profiles systemic clearance of OPCNs-PEG-FA nanoparticles from the host. Then, pharmacokinetics of OPCNs-PEG-FA nanoparticles was further performed using Kinetical 4.4 software (Thermo Fisher Scientific, Inc., America) for nonlinear least-squares analysis 57. The plasma concentration-time data was fitted to a bi-exponential equation as follows:
| C(t) = A1 * e-k1*t + A2 * e-k2*t | (6) |
where C(t) means the nanoparticles concentration at time t; A1 and A2 are the Y-intercepts; k1and k2 refer to the apparent first-order elimination rate constants 58. The area under curve (AUC) could be calculated from the sums of the ratios Al/kl and A2/k2. Afterwards, Clearance (CL) could be determined by dividing dose by AUC. The mean residence time (MRT) was calculated by following Equation 59:
| MRT = AUMC / AUC | (7) |
where AUMC is the area under the product of C*t plotted against t from time 0 to infinity. The initial half-life (t1/2), AUC and MRT of OPCNs-PEG-FA nanoparticles are summarized in Table S1. In terms of changes in t1/2, AUC, MRT and CL, compared to bare OPCNs, PEGylation of OPCNs demonstrated better fitting in vivo with longer half-life and retention time, larger area and slower elimination with respect to a previous study 60.
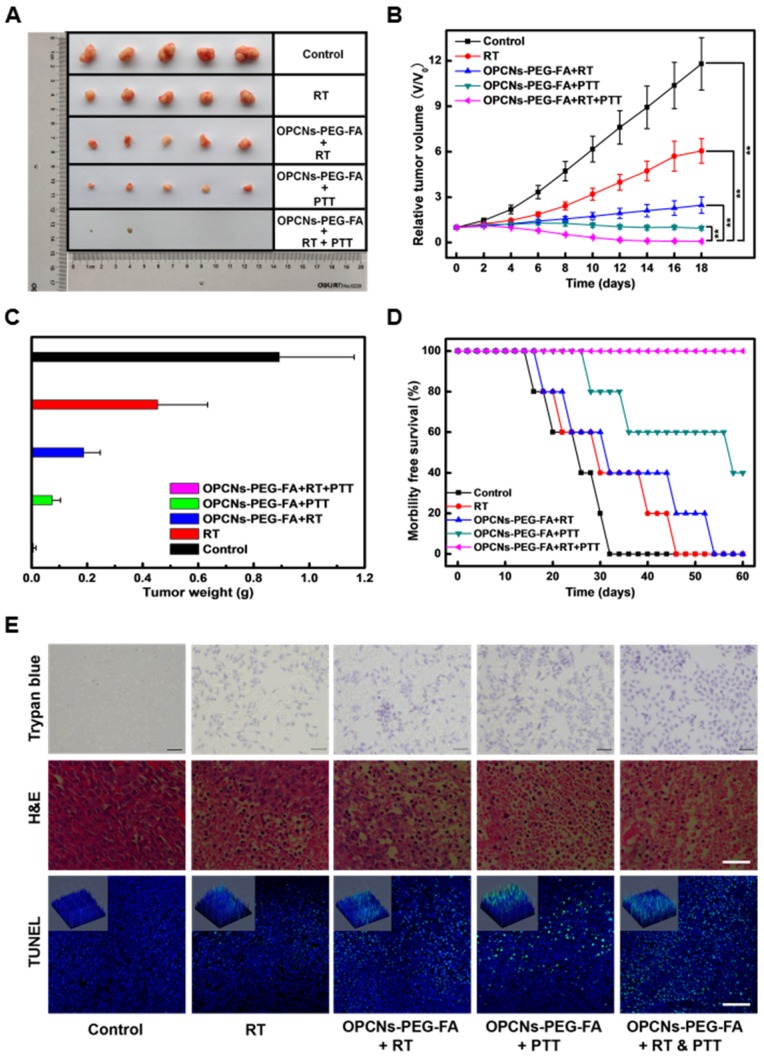
We further explored the therapeutic effect of OPCNs-PEG-FA in vivo. The tumor-bearing mice were randomly divided into five groups to receive different treatments: (a) control (PBS only); (b) RT only (120 kvp, 10 Gy, 10 min); (c) OPCNs-PEG-FA + RT (120 kvp, 10 Gy, 10 min); (d) OPCNs-PEG-FA + PTT (808nm, 2.4 W/cm2, 5 min); (e) OPCNs-PEG-FA + RT (120 kvp, 10 Gy, 10 min) + PTT (808nm, 2.4 W/cm2, 5 min). Subsequently, the relative tumor volumes of all these five groups were recorded as a function of time. Compared with control and single treatment groups (a, b, c and d), combinational photothermal radiotherapy led to nearly complete remission of tumor after 18 days' therapy (Fig. 7 A). In addition, as shown in Fig. 7 B, rapid tumor growth was observed in the control group. Meanwhile, it was found that group (b), (c) and (d) show different degrees of tumor inhibition, which could be attribute to the impotent anti-tumor efficacy of the individual RT, OPCNs-PEG-FA + RT or OPCNs-PEG-FA + PTT. As for the combination therapy group, the tumors were remarkably inhibited at the very beginning. On day 18, all tumors from all five different groups were collected and weighted (Fig. 7 C). Similarly, the combined photothermal radiotherapy displays much better therapeutic effect than any other single administration groups. Moreover, in this combined therapy group, survival rate of the mice was much higher than the control group and the single treatment groups (Fig. 7 D). Histological examinations with Trypan blue, H&E and TUNEL staining (Fig. 7 E) suggest that the combined photothermal radiotherapy resulted in more severe tumor ablation than other single treatment groups via inducing cell apoptosis or necrosis (green dots).
Figure 7.
In vivo evaluations of OPCNs-PEG-FA guided photothermal radiotherapy combination therapy. (A) Photographs of the tumors in different treatments groups for 18 days; (B) Relative tumor volumes and (C) weights of mice after different therapy administration; (D) Survival rates of tumor-bearing mice with in different treatment groups during 60 days; and (E) Apoptosis assay of tumor tissues with Trypan blue (Scale bar: 50 μm, dead cells were reflected by blue color with trypan blue staining), H&E (Scale bar: 100 μm) and TUNEL (Scale bar: 100 μm, Green: apoptosis DNA; Blue: cell nuclei) staining, respectively.
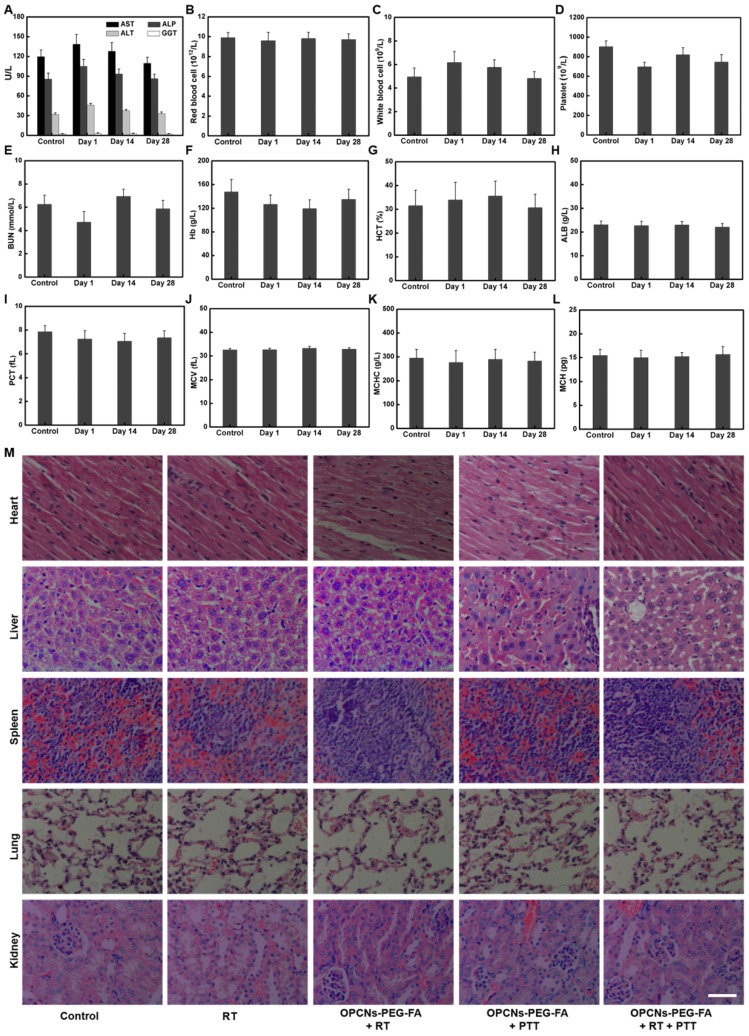
Finally, to ensure the practical feasibility of OPCNs-PEG-FA for potential clinic application, the hemocompatibility and in vivo toxicity of the OPCNs-PEG-FA nanoparticles was assessed by hemolytic ratio test 61, blood coagulation 62, blood biochemistry, hematology analysis, and histological examination (Table S2 and Fig. 8 A-L). In our study, after the i.v. injection of OPCNs-PEG-FA (100 mg/kg), most hematological parameters were found to be normal during the experimental period, compared with those of healthy mice in control group and groups with single therapy 63. Meanwhile, no obvious histopathological abnormalities were observed in major organs of OPCNs-PEG-FA nanoagents treated mice at the 28th day (Fig. 8 M). All preliminary toxicology results reveal that no apparent toxicity was caused by OPCNs-PEG-FA in vivo at the given dose within 28 d, suggesting that OPCNs-PEG-FA was a biocompatible theranostic nanoplatform for clinic use. Anyhow, further careful and meticulous studies are still needed to evaluate the long-term biodistribution behaviour and the metabolic mechanism of OPCNs-PEG-FA in vivo.
Figure 8.
In vivo toxicology examination for mice treated with OPCNs-PEG-FA. Hematology analysis and blood biochemistry of mice determined on the 1th, 14th and 28th day (n = 5). The examined items including (A) aspartate aminotransferase (AST), alkaline phosphatase (ALP), alanine aminotransferase (ALT), and gamma-glutamyltranspetidase (GGT); (B) Red blood cell (RBC) counts; (C) White blood cell (WBC) counts; (D) Platelets (PLT) counts; (E) Blood urea nitrogen (BUN); (F) Hemoglobin (HB); (G) Hematocrit (HCT); (H) Albumin (ALB) level; (I) Plateletcrit (PCT); (J) Mean corpuscular volume (MCV); (K) Mean corpuscular hemoglobin concentration (MCHC); (L) Mean corpuscular haemoglobin (MCH); and (M) Micrographs of H&E-stained tissue sections of major organs including heart, liver, spleen, lung, and kidney of healthy mice and mice treated with OPCNs-PEG-FA on the 28th day post the last injection of nanoparticles.
Taken together, we confirmed our hypothesis that OPCNs-PEG-FA nanoagents could efficiently inhibit tumor growth by synergistic photothermal radiotherapy. The nanoagents had good biocompatibility, hemocompatibility in vitro and in vivo. Our work provides a multifunctional theranostic nanoplatform with uniform morphology for dual-modal image-guided tumor therapy strategy. The enhanced RT and PTT nanoagents demonstrated good potential for clinical translation in tumor radio-photothermal ablation. Even though, the immunoreactions of OPCNs-PEG-FA along with photothermal radiotherapy need to be further investigated.
Conclusions
In this study, we designed and fabricated a biocompatible theranostic photothermal radiotherapy nanoplatform based on octopod Pt-Cu alloy nanoframes via a simple one-pot method, and then modified it with PEG and FA. The multifunctional nanotheranostics of OPCNs-PEG-FA achieved simultaneous ITI/PAI imaging and synergistic dual-modal RT/PTT tumor ablation. The as-synthesized nanoplatform of OPCNs-PEG-FA exhibited strong NIR light absorbance, good dispersity, hemocompatibility, biocompatibility and remarkable radio-sensitivity when exposed to X-ray, which could be used for multi-modality imaging and combined photothermal radiotherapy application for curing tumor. Meanwhile, the nanotheranostic agent not only improved synergistic tumor suppression, but also exhibited no obvious systemic toxicity in vivo.
Supplementary Material
Supplementary figures and tables.
Acknowledgments
This work was financially supported by National Natural Science Foundation of China (No. 31640030, U1404824), National Key R&D Program of China (Grant No. 2016YFC1100300), Visiting Scholar Foundation of Key Laboratory of Biorheological Science and Technology (Chongqing University), Ministry of Education (CQKLBST-2017-002) and Innovation Team in University of Chongqing Municipal Government (CXTDX201601002).
Abbreviations
- OPCNs
concave PtCu octopod nanoframes
- PEG
poly(ethylene glycol)
- FA
folic acid
- NIR
near-infrared
- ITI
infrared thermal imaging
- PAI
photoacoustic imaging
- PTT
photothermal therapy
- RT
radiotherapy
- ARS
acute radiation syndrome
- SEM
scanning electron microscopy
- TEM
transmission electron microscopy
- HAADF-STEM
high angle annular dark field scanning transmission electron microscopy
- EDX
energy dispersive X-ray
- EDS
energy dispersive X-ray spectroscopy
- XRD
X-ray powder diffraction
- XPS
X-ray photoelectron spectroscopy
- FTIR
Fourier-transform infrared spectroscopy
- DMEM
dulbecco's modified eagle medium
- FBS
fetal bovine serum
- NaN3/DOG
sodium azide/deoxyglucose
- Mβ-CD
methyl-β-cyclodextrin
- DMSO
Dimethyl sulfoxide
- ICP-AES
inductively coupled plasma atomic emission spectrometry
- HR
hemolysis ratio
- H&E
haematoxylin-eosin
- ANOVA
analysis of variance
- FCC
face-centered-cubic
- CLSM
confocal laser scanning microscopy
- FR
folic acid-receptor
- FR+
folic acid-receptor positive
- FR-
folic acid-receptor negative
- MTT
methyl thiazolyl tetrazolium
- SER
sensitivity enhancement ratio
- CI
combination index
- GI50
50% cell growth inhibition
- AUC
area under curve
- CL
clearance
- MRT
mean residence time
- AST
aspartate aminotransferase
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- GGT
gamma-glutamyltranspetidase
- RBC
Red blood cell
- WBC
White blood cell
- PLT
Platelets
- BUN
Blood urea nitrogen
- HB
Hemoglobin
- HCT
Hematocrit
- ALB
Albumin
- PCT
Plateletcrit
- MCV
Mean corpuscular volume
- MCHC
Mean corpuscular hemoglobin concentration (MCHC)
- MCH
mean corpuscular haemoglobin.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global Cancer Statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Chen G, Roy I, Yang C, Prasad PN. Nanochemistry and Nanomedicine for Nanoparticle-based Diagnostics and Therapy. Chem Rev. 2016;116:2826–2885. doi: 10.1021/acs.chemrev.5b00148. [DOI] [PubMed] [Google Scholar]
- 3.Bi S, Yue S, Zhang S. Hybridization Chain Reaction: A Versatile Molecular Tool for Biosensing, Bioimaging, and Biomedicine. Chem Soc Rev. 2017;46:4281–4298. doi: 10.1039/c7cs00055c. [DOI] [PubMed] [Google Scholar]
- 4.Zhang C, Zhao KL, Bu WO, Ni DL, Liu YY, Feng JW. et al. Marriage of Scintillator and Semiconductor for Synchronous Radiotherapy and Deep Photodynamic Therapy with Diminished Oxygen Dependence. Angew Chem Int Ed. 2015;127:1790–1794. doi: 10.1002/anie.201408472. [DOI] [PubMed] [Google Scholar]
- 5.Schaue D, McBride WH. Opportunities and Challenges of Radiotherapy for Treating Cancer. Nat Rev Clin Oncol. 2015;12:527–540. doi: 10.1038/nrclinonc.2015.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song GS, Chen YY, Liang C, Yi X, Liu JJ, Sun XQ. et al. Catalase-Loaded TaOx Nanoshells as Bio-Nanoreactors Combining High-Z Element and Enzyme Delivery for Enhancing Radiotherapy. Adv Mater. 2016;28:7143–7148. doi: 10.1002/adma.201602111. [DOI] [PubMed] [Google Scholar]
- 7.Xu YJ, Li A, Zhao CZ, Yang K, Chen XY, Li WW. Ultrasmall Semimetal Nanoparticles of Bismuth for Dual-Modal Computed Tomography/Photoacoustic Imaging and Synergistic Thermoradiotherapy. ACS Nano. 2017;25:3990–4001. doi: 10.1021/acsnano.7b00476. [DOI] [PubMed] [Google Scholar]
- 8.Yang Z, Song JB, Dai YL, Chen JY, Wang F, Lin LS. et al. Self-Assembly of Semiconducting-Plasmonic Gold Nanoparticles with Enhanced Optical Property for Photoacoustic Imaging and Photothermal Therapy. Theranostics. 2017;7:2177–2185. doi: 10.7150/thno.20545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang XD, Luo ZT, Chen J, Shen X, Song SS, Sun YM. et al. Ultrasmall Au(10-12)(SG)(10-12) Nanomolecules for High Tumor Specificity and Cancer Radiotherapy. Adv Mater. 2014;26:4565–4568. doi: 10.1002/adma.201400866. [DOI] [PubMed] [Google Scholar]
- 10.Zhang XD, Chen J, Min Y, Park GB, Shen X, Song SS. et al. Metabolizable Bi2Se3 Nanoplates: Biodistribution, Toxicity, and Uses for Cancer Radiation Therapy and Imaging. Adv Funct Mater. 2014;24:1718–1729. [Google Scholar]
- 11.Li A, Li X, Yu XJ, Li W, Zhao RY, An X. et al. Synergistic Thermoradiotherapy Based on PEGylated Cu3BiS3 Ternary Semiconductor Nanorods with Strong Absorption in the Second Near-infrared Window. Biomaterials. 2017;112:164–175. doi: 10.1016/j.biomaterials.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 12.Lv RC, Yang PP, He F, Gai SL, Li CX, Dai YL. et al. A Yolk-like Multifunctional Platform for Multimodal Imaging and Synergistic Therapy Triggered by A Single Near-infrared Light. ACS Nano. 2015;9:1630–1647. doi: 10.1021/nn5063613. [DOI] [PubMed] [Google Scholar]
- 13.Deng YY, Li ED, Cheng XJ, Zhu J, Lu SL, Ge CC. et al. Facile Preparation of Hybrid Core-shell Nanorods for Photothermal and Radiation Combined Therapy. Nanoscale. 2016;8:3895–3899. doi: 10.1039/c5nr09102k. [DOI] [PubMed] [Google Scholar]
- 14.Porcel E, Liehn S, Remita H, Usami N, Kobayashi K, Furusawa Y. et al. Platinum Nanoparticles: A Promising Material for Future Cancer Therapy? Nanotechnology. 2010;21:85103–85109. doi: 10.1088/0957-4484/21/8/085103. [DOI] [PubMed] [Google Scholar]
- 15.Guo ZQ, Zou YL, He H, Rao JM, Ji SS, Cui XN. et al. Bifunctional Platinated Nanoparticles for Photo-induced Tumor Ablation. Adv Mater. 2016;28:10155–10164. doi: 10.1002/adma.201602738. [DOI] [PubMed] [Google Scholar]
- 16.Song GS, Cheng L, Chao Y, Yang K, Liu Z. Emerging Nanotechnology and Advanced Materials for Cancer Radiation Therapy. Adv Mater. 2017;29:201700996. doi: 10.1002/adma.201700996. [DOI] [PubMed] [Google Scholar]
- 17.Zhou M, Zhao J, Tian M, Song SL, Zhang R, Gupta S. et al. Radio-Photothermal Therapy Mediated by a Single Compartment Nanoplatform Depletes Tumor Initiating Cells and Reduces Lung Metastasis in Orthotopic 4T1 Breast Tumor Model. Nanoscale. 2015;7:19438–19447. doi: 10.1039/c5nr04587h. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Zou LL, Wang H, He B, Zeng LJ, Tan T, Cao HQ. et al. Current Approaches of Photothermal Therapy in Treating Cancer Metastasis with Nanotherapeutics. Theranostics. 2016;6:762–772. doi: 10.7150/thno.14988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilroy KD, Ruditskiy A, Peng HC, Qin D, Xia YN. Bimetallic Nanocrystals: Syntheses, Properties, and Applications. Chem Rev. 2016;116:10414–10472. doi: 10.1021/acs.chemrev.6b00211. [DOI] [PubMed] [Google Scholar]
- 20.Li JH, Hu Y, Hou YH, Shen XK, Xu GQ, Dai LL. et al. Phase-change Material Filled Hollow Magnetic Nanoparticles for Cancer Therapy and Dual Modal Bioimaging. Nanoscale. 2015;7:9004–9012. doi: 10.1039/c5nr01744k. [DOI] [PubMed] [Google Scholar]
- 21.Han HS, Choi KY, Lee H, Lee M, An JY, Shin S. et al. Gold-Nanoclustered Hyaluronan Nano-Assemblies for Photothermally Maneuvered Photodynamic Tumor Ablation. ACS Nano. 2016;10:10858–10868. doi: 10.1021/acsnano.6b05113. [DOI] [PubMed] [Google Scholar]
- 22.Zhao H, Chao Y, Liu JJ, Huang J, Pan J, Guo WL. et al. Current Approaches of Photothermal Therapy in Treating Cancer Metastasis with Nanotherapeutics. Theranostics. 2016;6:1833–1843. doi: 10.7150/thno.14988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou M, Zhang R, Huang M, Lu W, Song SL, Melancon MP. et al. A Chelator-Free Multifunctional [64Cu]CuS Nanoparticle Platform for Simultaneous Micro-PET/CT Imaging and Photothermal Ablation Therapy. J Am Chem Soc. 2010;132:15351–15358. doi: 10.1021/ja106855m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou ZJ, Hu KW, Ma R, Yan Y, Ni B, Zhang YJ. et al. Cancer Therapy: Dendritic Platinum-Copper Alloy Nanoparticles as Theranostic Agents for Multimodal Imaging and Combined Chemophotothermal Therapy. Adv Funct Mater. 2016;33:5971–5978. [Google Scholar]
- 25.Zhu XM, Wan HY, Jia HL, Liu L, Wang JF. Porous Pt Nanoparticles with High Near-Infrared Photothermal Conversion Efficiencies for Photothermal Therapy. Adv Healthcare Mater. 2016;5:3165–3172. doi: 10.1002/adhm.201601058. [DOI] [PubMed] [Google Scholar]
- 26.Chen Q, Xu L, Liang C, Wang C, Peng R, Liu Z. Photothermal Therapy with Immune-adjuvant Nanoparticles Together with Checkpoint Blockadefor Effective Cancer Immunotherapy. Nat Commun. 2016;7:13193. doi: 10.1038/ncomms13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang C, Xu L, Liang C, Xiang J, Peng R, Liu Z. Immunological Responses Triggered by Photothermal Therapy with Carbon Nanotubes in Combination with Anti-CTLA-4 Therapy to Inhibit Cancer Metastasis. Adv Mater. 2014;26:8154–8162. doi: 10.1002/adma.201402996. [DOI] [PubMed] [Google Scholar]
- 28.Zhang JF, Zhang J, Li WY, Chen R, Zhang ZY, Zhang WJ. et al. Degradable Hollow Mesoporous Silicon/Carbon Nanoparticles for Photoacoustic Imaging-Guided Highly Effective Chemo-Thermal Tumor Therapy in Vitro and in Vivo. Theranostics. 2017;7:3007–3020. doi: 10.7150/thno.18460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li YJ, Quan FX, Zhu EB, Chen L, Huang Y, Chen CF. PtxCuy Nanocrystals with Hexa-pod Morphology and Their Electrocatalytic Performances Towards Oxygen Reduction Reaction. Nano Res. 2015;8:3342–3352. [Google Scholar]
- 30.Nie Y, Li L, Wei ZD. Recent Advancements in Pt and Pt-Free Catalysts for Oxygen Reduction Reaction. Chem Soc Rev. 2015;44:2168–2201. doi: 10.1039/c4cs00484a. [DOI] [PubMed] [Google Scholar]
- 31.Liu JN, Bu WB, Pan LM, Zhang SJ, Chen F, Zhou LP. et al. Simultaneous Nuclear Imaging and Intranuclear Drug Delivery by Nuclear-targeted Multifunctional Upconversion Nanoprobes. Biomaterials. 2012;33:7282–7290. doi: 10.1016/j.biomaterials.2012.06.035. [DOI] [PubMed] [Google Scholar]
- 32.Jing LJ, Shao S, Wang Y, Yang Y, Yue XL, Dai ZF. Hyaluronic Acid Modified Hollow Prussian Blue Nanoparticles Loading 10-hydroxycamptothecin for Targeting Thermochemotherapy of Cancer. Theranostics. 2016;6:40–53. doi: 10.7150/thno.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scialabba C, Puleio R, Peddis D, Varvaro G, Calandra P, Cassata G. et al. Folate Targeted Coated SPIONs As Efficient Tool for MRI. Nano Res. 2017;9:1–16. [Google Scholar]
- 34.Hou YH, Cai KY, Li JH, Chen XY, Lai M, Hu Y. et al. Effects of Titanium Nanoparticles on Adhesion, Migration, Proliferation, and Differentiation of Mesenchymal Stem Cells. Int J nanomedicine. 2013;8:3619–3630. doi: 10.2147/IJN.S38992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu XL, Hu JM, Tian J, Ge ZS, Zhang GY, Luo KF. et al. Polyprodrug Amphiphiles: Hierarchical Assemblies for Shape-regulated Cellular Internalization, Trafficking, and Drug Delivery. J Am Chem Soc. 2013;135:17617–17629. doi: 10.1021/ja409686x. [DOI] [PubMed] [Google Scholar]
- 36.Cai KY, Hou YH, Hu Y, Zhao L, Luo Z, Shi YS. et al. Correlation of the Cytotoxicity of TiO2 Nanoparticles with Different Particle Sizes on a Sub-200-nm Scale. Small. 2011;7:3026–3031. doi: 10.1002/smll.201101170. [DOI] [PubMed] [Google Scholar]
- 37.Conde J, Oliva N, Atilano M, Song HS, Artzi N. Self-assembled RNA-triple-helix Hydrogel Scaffold for MicroRNA Modulation in the Tumour Microenvironment. Nat Mater. 2016;15:353–363. doi: 10.1038/nmat4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zaki N, Tirelli N. Gateways for the Intracellular Access of Nanocarriers: A Review of Receptor-mediated Endocytosis Mechanisms and of Strategies in Receptor Targeting. Expert Opin Drug Del. 2010;7:895–913. doi: 10.1517/17425247.2010.501792. [DOI] [PubMed] [Google Scholar]
- 39.Yang K, Hu L, Ma XX, Ye SQ, Cheng L, Shi XZ. et al. Multimodal Imaging Guided Photothermal Therapy Using Functionalized Graphene Nanosheets Anchored with Magnetic Nanoparticles. Adv Mater. 2012;24:1868–1872. doi: 10.1002/adma.201104964. [DOI] [PubMed] [Google Scholar]
- 40.Zhang ZC, Luo ZM, Chen B, Wei C, Zhao J, Chen JZ. et al. One-Pot Synthesis of Highly Anisotropic Five-Fold-Twinned PtCu Nanoframes Used as a Bifunctional Electrocatalyst for Oxygen Reduction and Methanol Oxidation. Adv Mater. 2016;28:8712–8717. doi: 10.1002/adma.201603075. [DOI] [PubMed] [Google Scholar]
- 41.Hong W, Wang J, Wang EK. Facile Synthesis of PtCu Nanowires with Enhanced Electrocatalytic Activity. Nano Res. 2015;8:2308–2316. [Google Scholar]
- 42.Lee MJ, Lim SH, Ha JM, Choi SM. Green Synthesis of High Purity Mesoporous Gold Sponges Using Self-Assembly of Gold Nanoparticles Induced by Thiolated Poly(ethylene glycol) Langmuir. 2016;32:5937–5945. doi: 10.1021/acs.langmuir.6b01197. [DOI] [PubMed] [Google Scholar]
- 43.Liu J, Li ZZ, Yang XL, Liu WS, Wang BD, Zhu YH. et al. A High-performance Imaging Probe with NIR Luminescence and Synergistically Enhanced T1-T2 Relaxivity for in Vivo Hepatic Tumor Targeting, Multimodal Imaging. Chem Commun. 2015;51:13369–13372. doi: 10.1039/c5cc04911c. [DOI] [PubMed] [Google Scholar]
- 44.Jain V, Bharatam PV. Pharmacoinformatic Approaches to Understand Complexation of Dendrimeric Nanoparticles with Drugs. Nanoscale. 2014;6:2476–2501. doi: 10.1039/c3nr05400d. [DOI] [PubMed] [Google Scholar]
- 45.Lai M, Cai KY, Hu Y, Yang XF, Liu Q. Regulation of the Behaviors of Mesenchymal Stem Cells by Surface Nanostructured Titanium. Colloid Surface B. 2012;97C:211–220. doi: 10.1016/j.colsurfb.2012.04.029. [DOI] [PubMed] [Google Scholar]
- 46.Xu JJ, Xu BH, Shou D, Qin FH, Xu Y, Hu Y. Characterization and Evaluation of a Folic Acid Receptor-targeted Cyclodextrin Complex As an Anticancer Drug Delivery System. Eur J Pharm Sci. 2016;83:132–142. doi: 10.1016/j.ejps.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 47.Luo Z, Cai KY, Hu Yan, Zhang BL, Xu DW. Cell-Specifi c Intracellular Anticancer Drug Delivery from Mesoporous Silica Nanoparticles with pH Sensitivity. Adv Healthcare Mater. 2012;1:321–325. doi: 10.1002/adhm.201100030. [DOI] [PubMed] [Google Scholar]
- 48.Hu W, Qiu LP, Cheng L, Hu Q, Liu Y, Hu ZY. et al. Redox and pH Dual Responsive Poly (amidoamine) Dendrimer-poly (ethyleneglycol) Conjugates for Intracellular Delivery of Doxorubicin. Acta Biomater. 2016;36:241–253. doi: 10.1016/j.actbio.2016.03.027. [DOI] [PubMed] [Google Scholar]
- 49.Liu JJ, Luo Z, Zhang JX, Luo TT, Zhou J, Zhao XJ. et al. Hollow Mesoporous Silica Nanoparticles Facilitated Drug Delivery via Cascade pH Stimuli in Ttumor Microenvironment for Tumor Therapy. Biomaterials. 2016;83:51–65. doi: 10.1016/j.biomaterials.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 50.Xu WH, Han M, Dong Q, Fu ZX, Diao YY, Liu H. et al. Doxorubicin-mediated Radiosensitivity in Multicellular Spheroids from a Lung Cancer Cell Line is Enhanced by Composite Micelle Encapsulation. Int J Nanomedicine. 2012;7:2661–2671. doi: 10.2147/IJN.S30445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mao FX, Wen L, Sun CX, Zhang SH, Wang GL, Zeng JF. et al. Ultra-Small Biocompatible Bi2Se3 Nanodots for Multimodal-Imaging Guided Synergistic Radio-Photothermal Therapy against Cancer. ACS Nano. 2016;10:11145–11155. doi: 10.1021/acsnano.6b06067. [DOI] [PubMed] [Google Scholar]
- 52.Ma NN, Jiang YW, Zhang XD, Wu H, Myers JN, Liu PY. et al. Enhanced Radiosensitization of Gold Nanospikes via Hyperthermia in Combined Cancer Radiation and Photothermal Therapy. ACS Appl Mater Interfaces. 2016;8:28480–28494. doi: 10.1021/acsami.6b10132. [DOI] [PubMed] [Google Scholar]
- 53.Yi X, Chen L, Zhong XY, Gao RL, Qian YT, Wu F. et al. Cor-shell Au@MnO2 Nanoparticles for Enhanced Radiotherapy via Iimproving the Tumor Oxygenation. Nano Research. 2016;11:3267–3278. [Google Scholar]
- 54.Chou TC. Theoretical Basis, Experimental Design, and Computerized Simulation of Synergism and Antagonism in Drug Combination Studies. Pharmacol Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 55.Huang L, Jiang YY, Chen YZ. Predicting Drug Combination Index and Simulating the Network-Regulation Dynamics by Mathematical Modeling of DrugTargeted EGFR-ERK Signaling Pathway. Sci Rep. 2017;7:40752. doi: 10.1038/srep40752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li JH, Zhang FS, Hu ZG, Song WD, Li GD, Liang GF. et al. Drug “Pent-Up” in Hollow Magnetic Prussian Blue Nanoparticles for NIR-Induced Chemo-Photothermal Tumor Therapy with Trimodal Imaging. Adv healthcare Mater. 2017;6:201700005. doi: 10.1002/adhm.201700005. [DOI] [PubMed] [Google Scholar]
- 57.Yang Z, Leon J, Martin M, Harder JW, Zhang R, Liang D. et al. Pharmacokinetics and Biodistribution of Near-infrared Fluorescence Polymeric Nanoparticles. Nanotechnology. 2009;20:165101. doi: 10.1088/0957-4484/20/16/165101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mcguire KJ, Dewalle DR, Gburek WJ. Evaluation of Mean Residence Time in Subsurface Waters Using Oxygen-18 Fluctuations During Drought Conditions in the Mid-Appalachians. J Hydrol. 2002;261:132–149. [Google Scholar]
- 59.Gabizon A, Catane R, Uziely B, Kaufman B, Safra T, Cohen R. et al. Prolonged Circulation Time and Enhanced Accumulation in Malignant Exudates of Doxorubicin Encapsulated in Polyethylene-glycol Coated Liposomes. Cancer Res. 1994;54:987–992. [PubMed] [Google Scholar]
- 60.Verma MS, Liu SY, Chen YY, Meerasa A, Gu FX. Size-Tunable Nanoparticles Composed of Dextran-b-poly(D, L-lactide) for Drug Delivery Applications. Nano Res. 2012;5:49–61. [Google Scholar]
- 61.Cai KY, Li JH, Luo Z, Hu Y, Hou YH, Ding XW. β-Cyclodextrin Conjugated Magnetic Nanoparticles for Diazepam Removal from Blood. Chem Commun. 2011;47:7719–7721. doi: 10.1039/c1cc11855b. [DOI] [PubMed] [Google Scholar]
- 62.Biran R, Pond D. Heparin Coatings for Improving Blood Compatibility of Medical Devices. Adv Drug Deliv Rev. 2017;112:12–23. doi: 10.1016/j.addr.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 63.Wang SG, Li X, Chen Y, Cai XJ, Yao HL, Gao W. et al. A Facile One-Pot Synthesis of a Two-Dimensional MoS2/Bi2S3 Composite Theranostic Nanosystem for Multi-Modality Tumor Imaging and Therapy. Adv Mater. 2015;27:2775–2782. doi: 10.1002/adma.201500870. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures and tables.