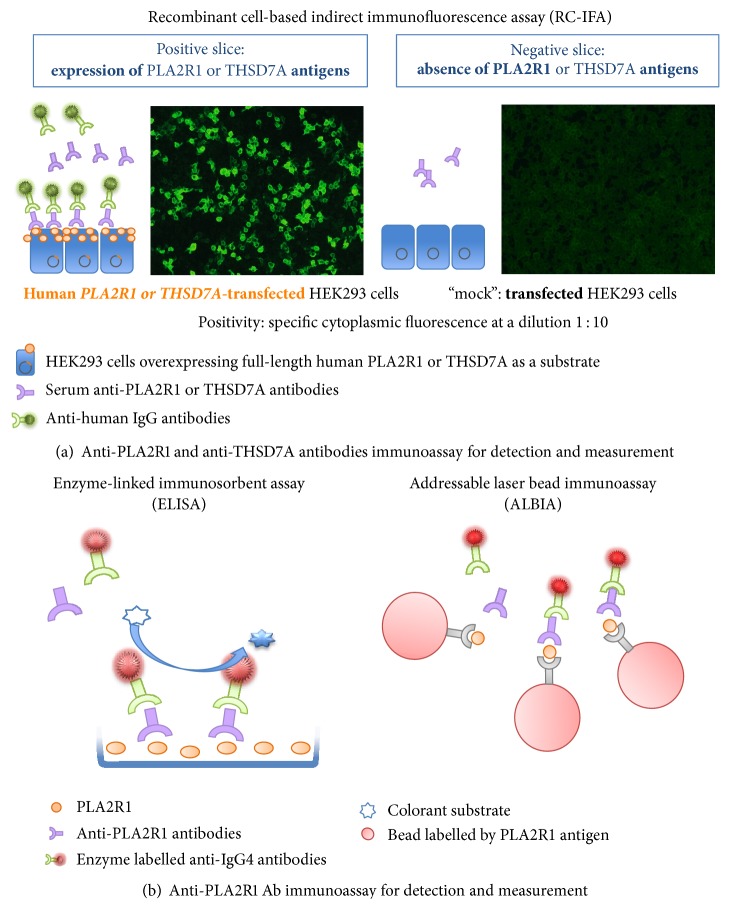
Figure 3.
Schematic presentations of available immunoassays detecting circulating anti-phospholipase 2 receptor 1 (PLA2R1) and anti-thrombospondin type 1 domain containing 7A (THSD7A) autoantibodies for diagnostic of primary membranous nephropathy (pMN). (a-b) Three standardized assays are currently available for diagnostic purposes of primary MN. The assessment of PLA2R1 could be performed using recombinant cell-based indirect immunofluorescence assay (RC-IFA), enzyme-linked immunosorbent assay (ELISA), or addressable laser bead immunoassay (ALBIA). The RC-IFA and ELISA are highly suitable for routine evaluation of pMN patients and are commercialized worldwide (Euroimmun AG, Luebeck, Germany). The ALBA developed by Mitogen Advanced Diagnostic Laboratory, Calgary, Canada, is a promising technique as it offers the possibility of analysing several antibodies in the same samples by one test. The RC-IFA uses the HEK293 human cell line overexpressing full-length human PLA2R1 protein. RC-IFA is a biochip format containing in one incubation field cells that express PLA2R1 antigen and control-transfected cells incapable of expressing PLA2R1. Using this test, anti-PLA2R1 antibodies are detected with very high specificity (nearly 100%) and high sensitivity (77%). Titers of anti-PLA2R1 antibodies decline during successful immunosuppressive therapy as well as during the spontaneous remission.