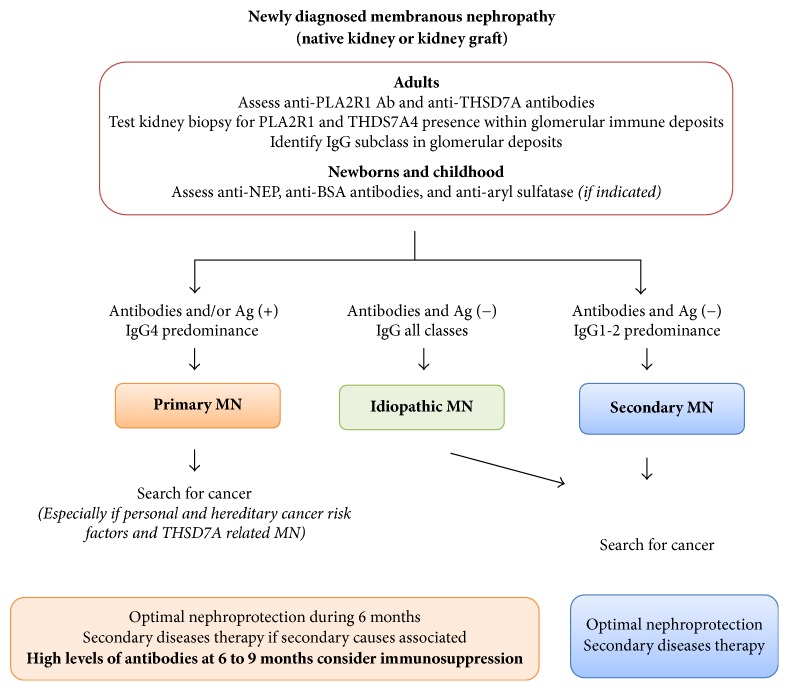
Figure 4.
Proposed summarized workup for diagnostic of membranous nephropathy in native kidney and kidney graft. We propose a revised clinical workup for patients with MN. The screening for the presence of circulating antibodies against PLA2R1 and THSD7A, useful new tools reflecting that autoimmune activity is essential for differentiating “pure pMN” from secondary MN. It is only necessary to test for anti-THSD7A in those patients who have apparently primary MN and who are negative for anti-PLA2R antibodies. Moreover, the routine investigation of kidney tissue biopsy needs to include the histological evaluation of glomerular immune deposits for PLA2R1, cationic BSA in childhood, and aryl sulfatase in patients under enzymes replacement therapy. Testing for THSD7A antigen is only required in cases with suspected primary MN that is negative for PLA2R1 antigen or who are suspected of having cancer-related MN. Those histological biomarkers are far more specific for pMN than the presence of less than 8 inflammatory cells per glomerulus. Under these circumstances further etiological investigations could by stopped except in patients with important personal and hereditary risk factors for cancer and management focused on optimal nephroprotective and immunosuppressive therapy. Intensive screening for secondary causes especially for the presence of malignancies is still advised in patients with THSD7A related MN. On the contrary, the absence of circulating anti-PLA2R1 antibodies and/or PLA2R1 antigen in association with IgG1 or IgG2 subclasses within extramembranous deposits is associated with an increased likelihood of secondary MN related to cancer, which should be thoroughly excluded by extensive investigation. In this setting, the treatment of secondary causes and optimal nephroprotection without immunosuppressive therapy has been advised. The overlap of pMN and secondary MN, especially in sarcoidosis or HBV infection, is possible. It could suggest postponing immunosuppression in patients with HBV infection and focusing on antiviral therapy. In this situation, monitoring of anti-PLA2R1 antibodies could be helpful to assess renal response. The levels of circulating antibodies need to be regularly monitored during the follow-up to evaluate the long-term outcome of disease, efficacy of therapeutic interventions, and the risk of disease relapse or of recurrence in recipients of kidney transplants. Adjustments of interventions in function of the evolution of antibody levels are likely to have a central role in individualizing care of patients with pMN in the future. Persistence over 6 to 9 months or increase in antibodies level associated with nephrotic proteinuria is a strong argument in favor of adjunction of immunosuppression to optimal supportive care. It would be logical to start when antibody levels are high to prevent worsening proteinuria and, on the contrary, to stop or at least taper the immunosuppressive treatment once the anti-PLA2R1 antibody levels are no longer detectable, which occurs in general months before urine protein levels decrease.