Abstract
Statement of the Problem:
A significant proportion of patients undergoing chemotherapy or radiotherapy suffer from mucositis. The first symptom of oral mucositis is pain. Severe pain, burning sensation, and discomfort in the oral cavity make it difficult to continue treatment and even continue living in these patients.
Purpose:
The aim of this study was to evaluate and compare the effect of amitriptyline mouthwash (in two forms of simple and niosomal) as a local anesthetic agent with benzydamine HCl mouthwash in oral mucositis after radiotherapy or chemotherapy.
Materials and Method:
This double-blind study was performed on 60 patients with oral mucositis caused by radiotherapy and chemotherapy. The severity of mucositis was determined based on patient judgment; then dental examination was performed and recorded in a checklist. Three groups were assigned based on using either benzydamine HCL, amitriptyline, or niosomal form of amitriptyline. Pain and burning sensation were evaluated with VAS at different time intervals: before use and one, five, ten, and thirty minutes and one hour after using mouthwash. T-test was used to compare the intensity of pain between the two groups. ANOVA and Tukey test were used to compare the intensity of pain between groups.
Results:
Statistical analyses showed the maximum reduction in pain intensity at two different time intervals (p= 0.04). Ten minutes after the use of niosomal form of amitriptyline, a 95% decrease in pain was observed. A 99% reduction in pain occurred after the use of simple form of amitriptyline (p= 0.04).
Conclusion:
Use of amitriptyline mouthwash had local anesthetic effects in oral mucositis without systemic side effects. Decrease in the severity of pain with the use of amitriptyline mouthwash was more than that of benzydamine HCL mouthwash.
Keywords: Amitriptyline , Benzydamine Mucositis , Topical anesthetic , Mouthwash
Introduction
Cancer is one of the most important health challenges. Based on WHO reports, diagnosis of new cancer cases is on the increase at a constant rate all over the world.[1] Currently, head and neck cancers are at the focus of attention in the dental field, particularly in Iran, where a large number of individuals have oral and oropharyngeal cancers.[2] Currently, techniques to treat cancer include surgery, radiotherapy, chemotherapy and a combination of these three techniques.[1,3] Generally, all the patients undergoing radiotherapy of the head and neck region exhibit oral complications.[4] The most common and debilitating complications of radiotherapy in patients with head and neck cancers are mucositis and xerostomia.[5] A significant proportion of patients undergoing chemotherapy are also affected by mucositis.[6] The first symptom of oral mucositis is pain.[7] Severe pain, burning sensation and discomfort makes it difficult for the patient to continue treatment and even continue living,[8] also resulting in weight loss or debility.[9] Mucositis lesions are painful; therefore, eating, speaking, and oral drug therapy becomes difficult due to pain, resulting in morbidity and mortality. This also increases the costs of health care and affects the quality of life of patients.[10-12] In addition, xerostomia can influence the quality of life in these patients.[13]
Currently, one of the most effective and common palliative treatments used for oral lesions and mucositis is the benzydamine mouthwash. An important consideration is the fact that the majority of such treatments relieve pain for a short time.[7] Amitriptyline is a tricyclic antidepressant drug that has receptors adjacent to the sodium channels in neurons that overlap the receptors of local anesthetic agents. This overlapping is independent from its antidepressant properties; such effect is observed when amitriptyline is prescribed topically, and contacts the oral mucosa.[14]
Epstein et al.[15] examined Doxepin mouthwash as a local anesthetic in patients with oral mucositis, caused by radiotherapy or bone marrow transplantation. Pain or burning sensation was evaluated with VAS for a week after using mouthwash. The pain reduced significantly after 5 minutes.[15] Moghaddamnia et al.[14] evaluated the effect of locally administered amitriptyline gel as an adjunct to local anesthetics in irreversible pulpitis pain by use of VAS at time intervals of 0, 1, 3, 5, 7 and 9 minutes after application of the gel. There was a 92.5% decrease in VAS scores of patients 9 minutes after amitriptyline administration compared to interval 0. Movassaghian et al.[16] examined mucoadhesive amitriptyline to control pain during dental treatment and concluded that mucoadhesive amitriptyline could be a good alternative to anesthetic regional channels. Movassaghian et al.[17] evaluated the clinical anesthetic effectiveness of intraoral mucoadhesive tablets of amitriptyline after infiltration in healthy volunteers. Pain sensation was evaluated with VAS at time intervals of 20, 25, 30 and 40 minutes after using intraoral mucoadhesive tablets of amitriptyline and concluded that amitriptyline is effective and successful in this way.[17]
Considering the extent of oral lesions and the pain resulting from them and the effect of such pain on the quality of life of patients, we decided to prepare a mouthwash with amitriptyline using the niosomal technique (slow release). This study aimed to evaluate and compare this mouthwash with benzydamine mouthwash in an attempt to relieve pain in oral lesions for longer periods during the healing period.
Materials and Method
In the present double-blind controlled clinical trial, the subjects consisted of 60 patients aged 14-74 years, with head and neck cancers, consisting of laryngeal and oral cancers, salivary gland tumors and bone marrow tumors. The subjects were selected from those referring to the Department of Oncology, Shafa Hospital, Kerman, for radiotherapy or to the Department of Hematology, Shahid Bahonar Hospital, Kerman, for chemotherapy. The inclusion criteria consisted of receiving a radiation dose of >3000 cGy and presence of at least, one major salivary gland, on one side in the radiotherapy field, and not being allergic to the tricyclic antidepressant drugs. In addition, the female subjects should have not been pregnant or breastfeeding; and the subjects should have not taken any analgesics or any local anesthetic agents, 4‒6 hours before the study. All subjects had pain or burning sensation in the oral cavity.[13]
The specialist in charge of the patients referred them for inclusion in the study when oral mucositis was diagnosed. The patients were referred only once and for only one hour. In order to prevent the patients from being deprived of their own principal and routine treatment, all the patients received their routine treatment for oral mucositis, rendered by their physician (Nystatin drop and diphenhydramine syrup as a mouthwash). The Ethics Committee of the Neurology Research Center, Kerman University of Medical Sciences, approved the protocol of the study under the code IRCT2015112123 529N1 and ethical code of K/93/366. All the subjects signed an informed consent form.
The subjects were selected consecutively and the type of the mouthwash was selected using simple random technique. First, the mucositis status was recorded in a checklist based on the description by the patient (patient‐judged mucositis grading). Then a dentist examined the oral cavity and recorded the mucositis status in another section of the checklist. In the present study, oral mucositis was defined as the inflammatory response of the oral cavity epithelium to radiotherapy and chemotherapy of cancer.[2] Two approaches were used in the study: physician‐judged mucositis grading and patient‐judged mucositis grading, which are described in Table 1 and Table 2. [18]
Table 1.
Patient- judged mucositis grading
| Grade | Definition |
|---|---|
| 0 (none) | None |
| 1 (mild) | Mild discomfort |
| 2 (moderate) | Definite discomfort but able to eat solid foods |
| 3 (severe) | Marked discomfort that interferes with eating solid foods |
| 4 (intravenous feeding) | Marked discomfort that prevents taking fluid or food by mouth, thus requiring intravenous feeding |
Table 2.
Physician‐judged mucositis grading
| Grade | Definition |
|---|---|
| 0 (none) | No toxicity |
| 1 (mild) | Painless ulcers, erythema, or mild soreness |
| 2 (moderate) | Painful erythema, edema, or ulcers but can eat |
| 3 (severe) | Painful erythema, edema, or ulcers and cannot eat |
| 4 (intravenous feeding) | Requires parenteral or enteral support |
Pharmacists made mouthwashes in the same containers. Niosome of amitriptyline was prepared by hydration of a thin layer of fat and combination with Brij family and cholesterol. Simple form of amitriptyline was prepared as a solution with 0.1% consternation of amitriptyline, and benzydamine HCL mouthwash was procured from the pharmaceutical market.
Patients were selected consecutively and randomly and divided into 3 groups. Type of treatment was selected consecutively and randomly. The subjects in the group 1 (n=20) received benzydamine HCL mouthwash; those in the group 2 (n=20) received niosomal amitriptyline mouthwash and those in the group 3 (n=20) received simple amitriptyline mouthwash. Both the patients and the dentist were blinded to the type of the mouthwash used. Each subject received 15 mL of the mouthwash for 30 seconds and asked to refrain from eating and washing the mouth for at least one hour. Then the severity of pain or burning sensation was determined and recorded using visual analog scale (VAS) before the use of mouthwashes (baseline) and at 1-, 5-, 10- and 30-minute and 1-hour intervals after the use of mouthwashes. The method used to complete the VAS was explained to the subjects and if they could not understand it, they were questioned about the severity of pain and burning sensation at the specified intervals and recorded.
Statistical analyses were carried out with SPSS 16. Descriptive statistics (medians, means, maximums, minimums, and standard deviations) were used to describe the samples. T-test was used to compare severity of pain between each two groups. ANOVA or post hoc Tukey test was used to compare severity of pain between several groups. Repeated-measures ANOVA were used to compare the severity of pain between different time intervals in each group. Chi-squared test was used to compare subjects in different groups separately. Statistical significance was set at p< 0.05.
The following formula was used to evaluate changes in VAS scores in all the three groups at different intervals; [19] ((V0-Vi)/V0) *100 where V0 is the VAS core at baseline and Vi is the VAS score at different intervals after using the mouthwash. Therefore, the percentages of changes relative to baseline were calculated at different intervals in different groups.
Results
The subjects consisted of 60 patients with mean age ± SD = 55.8±14.5 years (age range= 20‒80 years). The majority of the subjects were male (41 subjects, 68.3%). In relation to educational level, the majority of the subjects had elementary education (22 subjects, 37.9%), 20 subjects (34.5%) were illiterate, and the rest had higher educational levels. In addition, 88.9% of the subjects were married (Table 3). There were no significant differences between the three groups in gender distribution (chi-squared test, p= 0.02) and mean age (ANOVA, p< 0.05).
Table 3.
Demographic characteristics of the study population
| Variable | Number | Percentage | |
|---|---|---|---|
| Sex | Male | 41 | 68.3 |
| Female | 19 | 31.7 | |
| Marital status | Married | 53 | 88.3 |
| Single | 7 | 11.7 | |
| Education | Illiterate Education | 22 | 36.7 |
| Primary | 22 | 36.7 | |
| Below Diploma | 8 | 13.3 | |
| Diploma | 5 | 8.3 | |
| Universities | 3 | 5 | |
| Total | 60 | ||
Graphs 1 and 2 present the mucositis status based on patient description and evaluations carried out by the dentist separately for each mouthwash. As shown, the majority of the subjects in all the three groups complained of oral mucosal discomfort, with no significant differences in the severity of oral mucosal discomfort between the three groups (chi-squared test, p< 0.05). Figure 1 and Figure 2 present the mucositis status based on patient description and evaluations carried out. The examination of the oral mucosa by the dentist revealed ulceration and erythema in the oral mucosa in the majority of patients in all the three groups, with no significant differences (chi-squared test, p> 0.05).
Figure1.
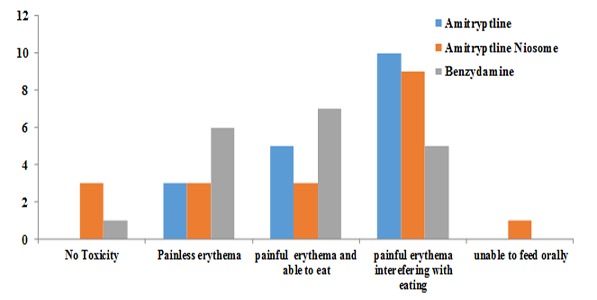
Physician grading
Figure2.
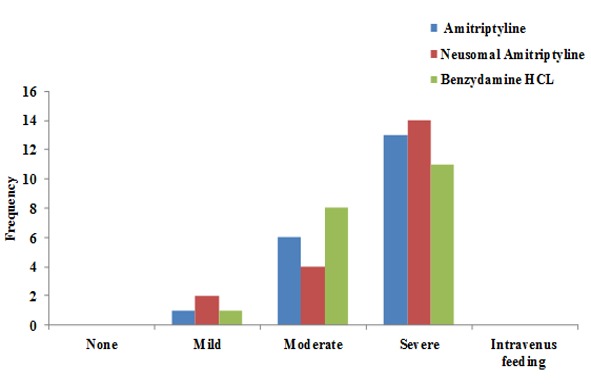
Patient‐judged mucositis grading
In order to evaluate the effect of mouthwashes used in the present study on the pain arising from mucositis, the patients’ pain severities were determined using VAS before using the mouthwash (q1), one minute (q2), 5 minutes (q3), 10 minutes (q4), 30 minutes (q5)- and 60 minutes (q6) after using the mouthwash.
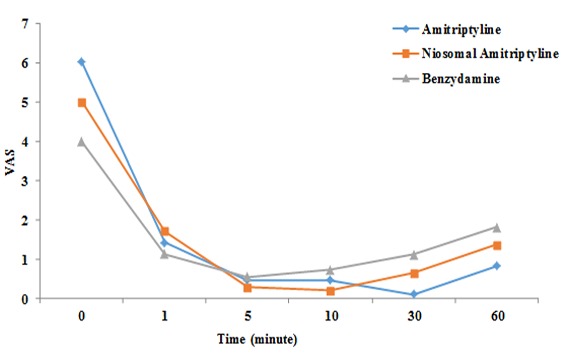
Figure 3 presents the mean VAS scores for all the three groups at different intervals. As shown in the Figure 4, in all groups, there were significant decreases in VAS scores after the use of mouthwashes (repeated-measures ANOVA, p= 0.004) (*p< 0.05 as compared to baseline, repeated-measures ANOVA followed by Tukey test). The results of Tukey test showed significant differences in q4 and q5 after the use of mouthwashes between three groups. In this context, at 10-minute interval, the niosomal formulation of amitriptyline and at 30-minute interval the simple form of amitriptyline mouthwash resulted in a more significant decrease in pain severity compared to others.
Figure3.
Mean VAS at various time intervals after use of the three mouthwashes
Figure4.
Percentage of reduction in pain intensity in the three groups at different times
Discussion
In all the three groups in the present study (amitriptyline mouthwash, niosomal form of amitriptyline and benzydamine mouthwash), there were significant decreases in VAS scores compared to baseline immediately after the use of the mouthwashes (p= 0.04). Movassaghian et al.[17] used amitriptyline as a mucoadhesive paste and reported significant decreases in pain severity at 20-, 25-, 30- and 40-minute intervals compared to placebo. Hoky et al.[20] used 5% amitriptyline paste topically on the skin for the treatment of neuropathic pains but did not report any significant and positive effects in comparison to lidocaine and placebo. Thompson et al.[21] carried out a systematic review and meta-analysis in relation to the topical use of amitriptyline on the skin for the treatment of neuropathy and reported that the advantage of topical use of amitriptyline was the absence of any systemic side effects; however, it had no positive effect on the relief of neuropathic pains. Nonetheless, several case reports were found that showed significant decreases in pain severity with the use of topical amitriptyline.[21]
In the present study, the maximum decrease in pain severity (99%) occurred with the use of simple from of amitriptyline mouthwash at 30-minute interval. In addition, with the use of the niosomal form of amitriptyline mouthwash there was a 92% decrease in pain severity at 10-minute interval and it was shown that the maximum decrease in pain severity occurred at this interval compared to the two other mouthwashes (p= 0.04). In a study by Moghaddamnia et al.,[14] % amitriptyline gel resulted in a 92.5% decrease in pain severity compared to placebo after 9 minutes. Epstein et al.[22] reported a 95% decrease in pain severity 15 minutes after the use of Doxepin mouthwash. In the present study, the maximum changes in pain severity relative to the baseline occurred in the amitriptyline mouthwash group. There were significant differences between groups at 10- and 30-minute intervals after using the mouthwashes, with the amitriptyline mouthwash resulting in a more significant decrease in pain severity compared to the niosomal form of amitriptyline and benzydamine (p= 0.04). It can be concluded that amitriptyline mouthwash has a higher anesthetic effect compared to the other two mouthwashes. It can also be concluded that amitriptyline mouthwash resulted in a more durable anesthetic effect compared with other two mouthwashes.
On the other hand, since the anesthetic effect of amitriptyline mouthwash at 30-minute interval was significantly higher than that of niosomal form of amitriptyline, it can be concluded that contrary to expectations, the niosomal form of amitriptyline did not have a more positive and better effect compared to amitriptyline mouthwash. Pain relapsed slowly in the majority of the subjects. Since most subjects still felt a decrease in pain severity at 60-minute interval compared to baseline, it can be concluded that if the evaluation period had been longer than 1 hour, better evaluation of the differences in the anesthetic effects of mouthwashes could be possible.
Epstein et al.[23] evaluated the anesthetic effect of Doxepin mouthwash at long time intervals and showed that this mouthwash significantly preserved its anesthetic effect up to 4 hours in healthy subjects and up to 3 hours in subjects with mucositis. Several studies have shown that the topical use of antidepressive agents, especially tricyclic agents (TCA) such as amitriptyline and Doxepin, decrease pain severity and lead to local anesthesia.[15,17,22-23] Wahl et al.[24] carried out a systematic review and reported that TCAs can be used as local anesthetic agents. Movassaghian et al.[16] used amitriptyline mucoadhesive paste to control pain during injection of anesthetic agents in dentistry and reported that amitriptyline could be used as a local anesthetic agent. Amitriptyline primarily exerts its effect as an inhibitor of the reabsorption of serotonin-norepinephrine in the brain. In addition, it has a sedative effect through the inhibition of H2 receptors.[17] Prescription of oral amitriptyline 90 minutes before surgery can also have supplementary anesthetic effects.[25] However, such an effect is exerted through the central nervous system and enkephalins.[25] Therefore, due to the central effects of amitriptyline in the painful nerve tracts, it has also been used as an analgesic in neuropathies caused by herpes zoster infections and in association with other medications in the oral burning sensation syndrome.[26] The effects of amitriptyline on neuropathies mentioned above can be explained by its effects on the descending serotonergic pathways in the posterior horns of the spinal cord and its effect on endorphin systems.[25] On the other hand, studies have shown that amitriptyline has binding sites similar to those of local anesthetic agents in sodium channels. These specific binding sites overlap with those of the local anesthetic agents.[14] In other words, amitriptyline might have binding sites similar to those of the local anesthetic agents in sodium channels or the binding sites might be very close together in terms of location and function.
Studies on rats have shown that at a similar concentration of lidocaine and amitriptyline, the duration of anesthesia induced by amitriptyline, was longer; hence, the researchers concluded that amitriptyline could be used as a local anesthetic agent.[27] The only study on the anesthetic effect of amitriptyline in the oral cavity was carried out in 2009,[14] in which amitriptyline gel was used to decrease pain due to irreversible pulpitis and the results showed its efficacy.[14] In addition, it has been reported that application of one drop of 10 mmol/L of amitriptyline on the tongue results in the anesthesia of that site for one hour.[28]
New drug delivery systems such as liposomes have been used for the sustained release, taste masking, or increasing stability of local anesthetic drugs such as lidocaine[29] and bupivacaine.[30] On the other hand, to increase the chemical stability and reduce the price, liposome systems such as niosomes that are a mixture of cholesterol and nonionic surfactants are employed.[31] In addition, ease of storage and plurality of nonionic surfactants available to provide resulted in preferring niosome to liposome in pharmaceutical products and cosmetics.
In the present study, amitriptyline was used for the first time in the form of a mouthwash as a local anesthetic agent to relieve pain due to mucositis and compared with benzydamine, which is routinely used in mucositis cases. Physicians prescribe different medications to relieve pain caused by mucositis, including diphenhydramine, lidocaine, and so on,[10-11] with benzydamine being the most common one. An important consideration is the fact that the majority of these treatments relieve pain for a short time.[7]
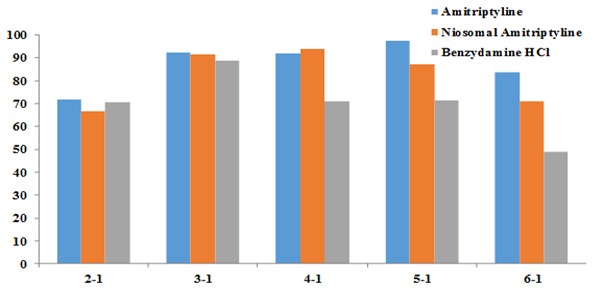
The majority of the subjects in the present study were hospitalized for chemotherapy and some were met during the waiting period for radiotherapy. The patients were followed for only 60 minutes after using the mouthwash. The mean decreases in pain intensity at 60-minute intervals after using the amitriptyline mouthwash was about 85%, 72% in the niosomal form of amitriptyline, and about approximately 50% with benzydamine mouthwash. However, the differences were not statistically significant. It appears that periods longer than an hour would have shown the efficacy of anesthesia provided by amitriptyline. Therefore, it is recommended that in future studies, evaluations should be carried out at longer periods.
Conclusion
Use of amitriptyline mouthwash has local anesthetic effects on the oral mucosa. Decrease in the severity of pain with the use of amitriptyline mouthwash was more than that with benzydamine mouthwash.
Footnotes
Conflict of interests: The authors of this manuscript certify that they have no conflict of interest.
References
- 1.Ashktorab T, Yazdani Z, Mojab F, Majd H, Madani H. Preventive effects of an oral rinse peppermint essence on chemotherapy-induced oral mucosistis. Koomesh. 2010; 12:Pe8–Pe13. [Google Scholar]
- 2.Kakoei S, Ghassemi A, Nakhaee N. Effect of cryotherapy on oral mucosis in patients with head and neck cancers receiving radiotherapy. Int J Radiat Res. 2013; 11: 117–120. [Google Scholar]
- 3.Talaipour A, Hadad P, Sahba S, Bashizadeh Fakhar H, Sakhdari S. Chamomile Mouth Rinse Effects on Mucositis Reduction After Radiotherapy. JDM. 2000; 13: 57–62. [Google Scholar]
- 4.Devita VT, Samuel JR, Rosenberg SA. Oral complications In: Berger AM, Kilroy TJ, editors: Cancer. 6th ed. Philadelphia: Lippicncott Williams and Wilkins; 2001. p. 2881. [Google Scholar]
- 5.Brosky ME. The role of saliva in oral health: strategies for prevention and management of xerostomia. J Support Oncol. 2007; 5: 215–225. [PubMed] [Google Scholar]
- 6.Shiboski CH, Hodgson TA, Ship JA, Schiødt M. Management of salivary hypofunction during and after radiotherapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007; 103 Suppl: S66.e1–19. doi: 10.1016/j.tripleo.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Rajesh VL, Stephen TS, Douglas EP. Management of oral mucositis in patients with cancer. J Dent Clin North Am. 2008; 52: 61–77. doi: 10.1016/j.cden.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epstein J, Van Der Vaal I. Oral cancer In: Burket’s oral medicine. 11th ed. BC Decker Inc: Hamilton; 2008. pp. 153–190. [Google Scholar]
- 9.Nagao Y, Sata M. Effect of oral care gel on the quality of life for oral lichen planus in patients with chronic HCV infection. Virol J. 2011; 8: 348. doi: 10.1186/1743-422X-8-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lalla RV, Sonis ST, Peterson DE. Management of oral mucositis in patients who have cancer. Dent Clin North Am. 2008; 52: 61–77. doi: 10.1016/j.cden.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubenstein EB, Peterson DE, Schubert M, Keefe D, McGuire D, Epstein J, et al. Clinical practice guidelines for the prevention and treatment of cancertherapy-induced oral and gastrointestinal mucositis. Cancer. 2004; 100(9 Suppl): 2026–2046. doi: 10.1002/cncr.20163. [DOI] [PubMed] [Google Scholar]
- 12.Aghamohamamdi A, Hosseinimehr SJ. Natural Products for Management of Oral Mucositis Induced by Radiotherapy and Chemotherapy. Integr Cancer Ther. 2016; 15: 60–68. doi: 10.1177/1534735415596570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kakoei S, Haghdoost AA, Rad M, Mohammadalizadeh S, Pourdamghan N, Nakhaei M, et al. Xerostomia after radiotherapy and its effect on quality of life in head and neck cancer patients. Arch Iran Med. 2012; 15: 214–218. [PubMed] [Google Scholar]
- 14.Moghadamnia AA, Partovi M, Mohammadianfar I, Madani Z, Zabihi E, Hamidi MR, et al. Evaluation of the effect of locally administered amitriptyline gel as adjunct to local anesthetics in irreversible pulpitis pain. Indian J Dent Res. 2009; 20: 3–6. doi: 10.4103/0970-9290.49047. [DOI] [PubMed] [Google Scholar]
- 15.Epstein JB, Epstein JD, Epstein MS, Oien H, Truelove EL. Doxepin rinse for management of mucositis pain in patients with cancer: one week follow-up of topical therapy. Spec Care Dentist. 2008; 28: 73–77. doi: 10.1111/j.1754-4505.2008.00015.x. [DOI] [PubMed] [Google Scholar]
- 16.Movassaghian S, Barzegar-Jalali M, Alaeddini M, Hamedyazdan S, Afzalifar R, Zakeri-Milani P, et al. Development of amitriptyline buccoadhesive tablets for management of pain in dental procedures. Drug Dev Ind Pharm. 2011; 37: 849–854. doi: 10.3109/03639045.2010.546403. [DOI] [PubMed] [Google Scholar]
- 17.Movassaghian S, Afzalifar R, Alaeddini M. Clinical anesthetic effectiveness of intraoral mucoadhesive tablets of amitriptyline in healthy volunteers. J Oral Maxillofac Surg. 2013; 71: 23–28. doi: 10.1016/j.joms.2012.08.029. [DOI] [PubMed] [Google Scholar]
- 18.Karagözoğlu S, Filiz Ulusoy, M Chemotherapy: the effect of oral cryotherapy on the development of mucositis. J Clin Nurs. 2005; 14: 754–765. doi: 10.1111/j.1365-2702.2005.01128.x. [DOI] [PubMed] [Google Scholar]
- 19.Vickers AJ. The use of percentage change from baseline as an outcome in a controlled trial is statistically inefficient: a simulation study. BMC Med Res Methodol. 2001; 1: 1–6. doi: 10.1186/1471-2288-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho KY, Huh BK, White WD, Yeh CC, Miller EJ. Topical amitriptyline versus lidocaine in the treatment of neuropathic pain. Clin J Pain. 2008; 24: 51–55. doi: 10.1097/AJP.0b013e318156db26. [DOI] [PubMed] [Google Scholar]
- 21.Thompson DF, Brooks KG. Systematic review of topical amitriptyline for the treatment of neuropathic pain. J Clin Pharm Ther. 2015;40:496–503. doi: 10.1111/jcpt.12297. [DOI] [PubMed] [Google Scholar]
- 22.Epstein JB, Epstein JD, Epstein MS, Oien H, Truelove EL. Oral doxepin rinse: the analgesic effect and duration of painreduction in patients with oral mucositis due to cancer therapy. Anesth Analg. 2006; 103: 465–470. doi: 10.1213/01.ane.0000223661.60471.78. [DOI] [PubMed] [Google Scholar]
- 23.Epstein JB, Truelove EL, Oien H, Allison C, Le ND, Epstein MS. Oral topical doxepin rinse: analgesic effect in patients with oral mucosalpain due to cancer or cancer therapy. Oral Oncol. 2001; 37: 632–637. doi: 10.1016/s1368-8375(01)00005-7. [DOI] [PubMed] [Google Scholar]
- 24.Wahl MJ, Brown RS. Dentistry's wonder drugs: local anesthetics and vasoconstrictors. Gen Dent. 2010; 58: 114–123. [PubMed] [Google Scholar]
- 25.Honda M, Nishida T, Ono H. Tricyclic analogs cyclobenzaprine, amitriptyline and cyproheptadineinhibit the spinal reflex transmission through 5-HT(2) receptors. Eur J Pharmacol. 2003; 458: 91–99. doi: 10.1016/s0014-2999(02)02735-8. [DOI] [PubMed] [Google Scholar]
- 26.Grushka M, Epstein JB, Gorsky M. Burning mouth syndrome and other oral sensory disorders: a unifyinghypothesis. Pain Res Manag. 2003; 8: 133–135. doi: 10.1155/2003/654735. [DOI] [PubMed] [Google Scholar]
- 27.Gerner P, Mujtaba M, Sinnott CJ, Wang GK. Amitriptyline versus bupivacaine in rat sciatic nerve blockade. Anesthesiology. 2001; 94: 661–667. doi: 10.1097/00000542-200104000-00021. [DOI] [PubMed] [Google Scholar]
- 28.Martin MD, Ramsay DS, Whitney C, Fiset L, Weinstein P. Topical anesthesia: differentiating the pharmacological and psychological contributions to efficacy. Anesth Prog. 1994; 41: 40–47. [PMC free article] [PubMed] [Google Scholar]
- 29.Franz-Montan M, Baroni D, Brunetto G, Sobral VR, da Silva CM, Venâncio P, et al. Liposomal lidocaine gel for topical use at the oral mucosa: characterization, in vitro assays and in vivo anesthetic efficacy in humans. J Liposome Res. 2015; 25: 11–19. doi: 10.3109/08982104.2014.911315. [DOI] [PubMed] [Google Scholar]
- 30.Ferlas B, Born M. Liposomal Bupivacaine: A New Option for Postoperative Pain. US Pharm. 2015; 40: HS17–HS20. [Google Scholar]
- 31.Pardakhty A, Moazeni E. Nano-niosomes in drug, vaccine and gene delivery: a rapid overview. Nanomedicine Journal. 2013; 1: 1–12. [Google Scholar]


