Figure 1.
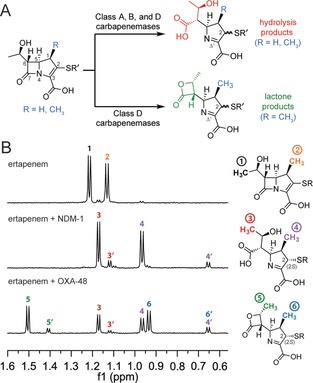
A) Major carbapenem‐derived products formed by β‐lactamases. The C‐2 stereochemistry of the products may depend on the particular enzyme. B) NMR spectra (600 MHz) showing the different product profiles of ertapenem (1 mm) with NDM‐1 (5 μm) or OXA‐48 (5 μm). Note that the minor peaks represent products with an assigned R configuration at C‐2 (indicated with a prime symbol; see main text and Figures S2–S5).
