Figure 1.
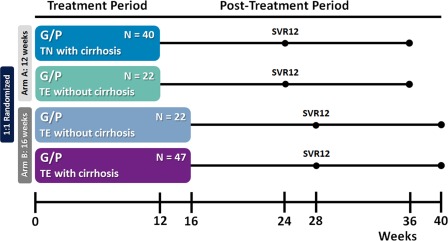
SURVEYOR‐II, Part 3 study design. Patients were enrolled into two arms to be treated with either 12 weeks (arm A) or 16 weeks (arm B) of G/P. Treatment‐naive patients with cirrhosis received 12 weeks of treatment, while those with prior treatment experience and cirrhosis received 16 weeks. Patients with prior treatment experience but without cirrhosis were randomized 1:1 for treatment with either 12 or 16 weeks of G/P. Patients were followed for 24 weeks posttreatment to monitor safety and sustained virologic response. Abbreviations: TE, treatment‐experienced; TN, treatment‐naive.
