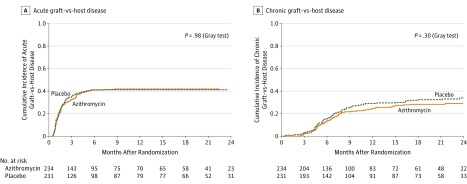
Figure 3. Graft-vs-Host Disease .
In this modified intention-to-treat analysis set, data cutoff was April 26, 2017.
A, The median (interquartile range [IQR]) follow-up for the occurrence of acute graft-vs-host disease in the azithromycin group was 8.5 months (IQR, 1.4-17.6) and 3.7 months (IQR, 1.4- 18.9) in the placebo group. Acute graft-vs-host disease includes symptoms of erythema, maculopapular rash, nausea, vomiting, anorexia, profuse diarrhea, ileus, or cholestatic liver disease.
B, The median (IQR) follow-up for the occurrence of chronic graft-vs-host disease, 7.1 months (IQR, 4.2-18.6) for the azithromycin group and was 7.9 months (IQR, 4.5-21.0) for the placebo group. Chronic graft-vs-host disease is a syndrome of variable clinical features resembling autoimmune and other immunologic disorders such as scleroderma, Sjögren syndrome, primary biliary cirrhosis, wasting syndrome, bronchiolitis obliterans, immune cytopenias, and chronic immunodeficiency; manifestations of chronic graft-vs-host disease may be restricted to a single organ or site or may be widespread.