Figure 4.
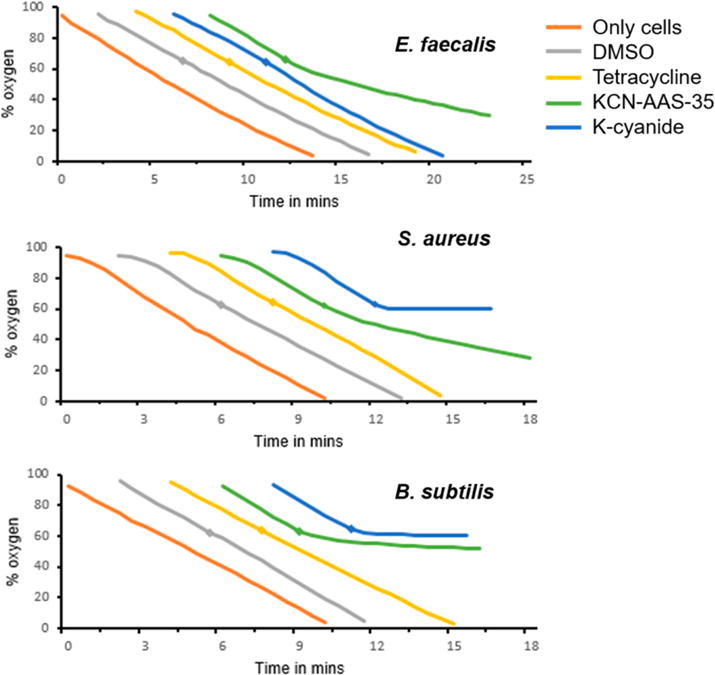
Oxygen consumption assays in the three bacterial strains show that KCN-AAS-35 inhibits cellular respiration. Aerobic respiration in E. faecalis S613, S. aureus MRSA 131, and B. subtilis 168 was measured in an oxygen electrode chamber by recording the percentage of extracellular oxygen remaining in the chamber as a function of time after addition of cells. The intrinsic respiration rate of the cells was recorded in the absence of any compounds (orange trace). Oxygen consumption was then measured by the addition of 12.5 μL of DMSO (gray trace), 105 μg/mL tetracycline (yellow trace), 10 mM potassium cyanide (blue trace), or 62.5 μg/mL KCN-AAS-35 (green trace), with the addition being made when 60% oxygen remained in the chamber (represented by diamonds on the respective traces). All assays were done in triplicate. The addition of DMSO (vehicle) or tetracycline did not affect bacterial respiration. The addition of cyanide, a known respiratory inhibitor, aborted respiration in S. aureus and B. subtilis but not in E. faecalis, which uses a cyanide insensitive cytochrome bd terminal oxidoreductase. The addition of KCN-AAS-35 caused a decrease in the rate of respiration in all the bacterial strains, with the effect being more pronounced in B. subtilis.
