Abstract
Despite biological rationale and significant clinical study, the pursuit of small-molecule kinase inhibitors for the treatment of brain cancers has had very limited success. This Advance-in-Brief discusses the need for drugs to achieve free brain penetration to engage their targets where CNS tumors reside. This need to achieve free, as opposed to total, drug concentrations in the brain may be a contributing factor to why so many small-molecule kinase inhibitors have not realized success in the neuro-oncology setting. For kinase targets of interest for brain cancer, either the vast majority of small-molecule inhibitors have data suggesting that free brain penetration would be limited or there are inadequate data to suggest that free brain penetration could be expected. Therefore, kinase targets of interest in the treatment of brain cancers may be inadequately assessed due to a lack of freely brain-penetrant inhibitors available for clinical study. Encouraging recent drug discovery efforts that focused on achieving free brain penetration for cancers in the CNS are highlighted. Still, further efforts are needed to enable thorough clinical evaluation of biological hypotheses.
Keywords: free brain penetration, free drug hypothesis, small molecule kinase inhibitors
For those conducting drug discovery research, the pages of this journal are a continuous reminder of the need for new treatment options for patients with brain cancers. From aggressive primary brain tumors, like glioblastoma multiforme (>12000 new diagnoses annually in the US), to the more than 150000 cases of brain metastases each year in the US, there are unmet medical needs of a magnitude that pharmaceutical discovery efforts should not ignore.1,2
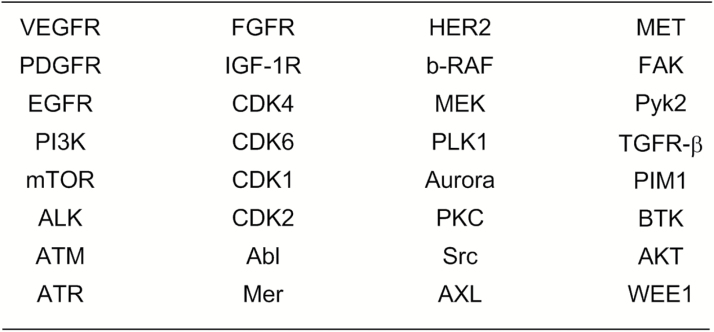
Medical need, strength of a biological hypothesis, market size, competitive landscape coupled with feasibility of discovery and development of a suitable drug are considerations that factor into whether a company pursues a drug discovery effort. For brain cancers the medical need is abundantly evident and there is no shortage of hypothesized drug targets amenable to inhibition by small-molecule drugs. Indeed, among the targets hypothesized as potential drivers of brain tumors are several kinases, a target class with demonstrated feasibility in drug discovery and development. A review of the literature reveals more than 2 dozen kinase targets that have been suggested as targets of interest for the treatment of brain cancers and for which inhibitors have advanced to clinical study (Figure 1).3 This set of kinases consists of some for which inhibitors would be of interest in a primary brain tumor setting, some of interest where brain metastases have emerged, and others that may be of interest in both settings. Of course, some of the targets may have greater merit as legitimate drug targets than others.
Fig. 1.
Kinases for which a biological rationale exists to target for brain cancers. For a discussion of inhibitors, see Heffron.3
Kinases have been the subject of intense interest both by pharmaceutical companies and by academic institutions for more than 30 years.4 Through 2016, as a result of these efforts, 33 small-molecule kinase inhibitors have been approved as treatments for cancer.5–7 Despite this apparent success, FDA approvals of kinase inhibitors for brain cancers are limited to everolimus, for the treatment of tuberous sclerosis, and alectinib, with accelerated approval, for use that includes in patients with brain metastases (anaplastic lymphoma kinase + non-small-cell lung carcinoma).8,9 Further, while there are approvals of numerous small-molecule kinase inhibitors whose primary targets are hypothesized as of interest for neuro-oncology (eg, epidermal growth factor receptor [EGFR], vascular endothelial growth factor receptor [VEGFR] inhibitors), there is a notable lack of regulatory approvals for such inhibitors to treat brain cancers.
The lack of regulatory drug approvals for kinase inhibitors in the brain cancer setting may not reflect that the biological rationales are flawed or invalid. It is quite possible that many of the drugs tested have not been able to sufficiently reach their target in CNS tumors, resulting in the biological hypotheses remaining untested. In order to expect efficacy from a small-molecule kinase inhibitor in any cancer setting, it must be able to engage its target within the tumor. In order for a drug to engage its target within a tumor in the CNS, it must first penetrate the blood–brain barrier (BBB). While there may be disruption of the BBB by some tumors, there is a growing body of literature suggesting that the barrier remains intact for some portion of the tumor or metastasis.10–19 Glioblastoma, for instance, is a highly diffusive, infiltrative disease and in most cases much of the tumor still has an intact BBB, preventing non-brain-permeable drugs from reaching the target.15–19 Brain metastases generally are less diffuse in comparison; however, the level of BBB disruption, and thus drug exposure in the brain tumor, is highly variable both between and within metastases.20 As an example, within human epidermal growth factor receptor 2–positive breast cancer brain metastases, lapatinib concentration within brain metastases was found to vary from 21% to 700% of serum concentrations.21 While individual patients might find benefit from a drug because of partial BBB disruption, many patients may not have this disruption and, for those who do, eventually tumor may progress behind intact BBB. Therefore, it is not reasonable to depend on BBB disruption to achieve consistent and sustained clinical responses, and drugs that (highly) penetrate the BBB are needed. Highlighting the fundamental requirement of free CNS penetration in the neuro-oncology setting is the emergence of CNS metastases in which the BBB provides a sanctuary from drug that is effectively treating a peripheral tumor. As examples, CNS metastases emerge as resistance to drugs known to ineffectively penetrate the BBB, including pertuzumab22 and the small-molecule kinase inhibitor crizotinib.23,24
To have potential for consistent and sustained clinical response in brain tumors, drugs must penetrate the BBB. This means that some assessment of BBB penetration should be conducted preclinically to determine the merits of clinical study. In preclinical assessments of brain penetration for drug candidates, total (as opposed to free) brain concentrations or total brain-to-plasma concentration ratios have historically been utilized to determine the extent to which a molecule penetrates the BBB. However, in assessing a molecule’s BBB penetration, simply measuring total drug concentrations is not sufficient to assess the level of brain penetration likely required for target engagement. The free drug hypothesis posits that it is the “free” drug (ie, the concentration of drug not nonspecifically bound to proteins or lipids) that is available to engage its target.25,26 This means that rather than assessing total drug concentrations in the brain, research investigators should correct for nonspecific binding to brain tissue to determine if a potentially effective, or “free,” drug concentration can be attained. Without this readily determined correction factor applied, an inaccurate picture of the potential of a drug candidate can result. For small-molecule drug discovery programs directed toward molecules with CNS penetration, we consider free brain-to-plasma ratio values >0.3 to demonstrate a significant degree of free CNS penetration. This minimal value for significance is chosen to increase confidence that the penetration measured is meaningful beyond potential experimental error. Furthermore, this target value of >0.3 exceeds the extracellular space in the brain.27 In the author’s experience with small-molecule kinase inhibitors, the total brain concentration or total brain-to-plasma concentration ratio is frequently much higher than the corresponding, and most meaningful, free drug values.
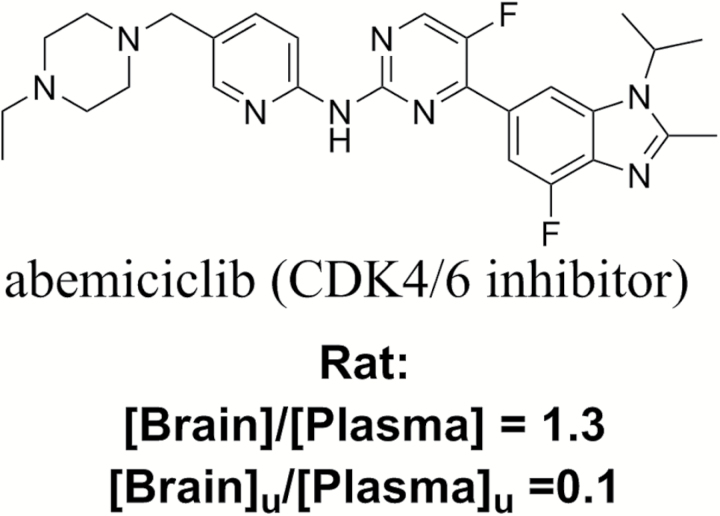
Abemaciclib serves as an illustrative example of how consideration of total brain-to-plasma concentration and free brain-to-plasma concentration ratios results in different outcomes in the assessment of brain penetration for a small-molecule kinase inhibitor (Figure 2). Researchers at Eli Lily describe a rat pharmacokinetic study of abemaciclib in which the total brain-to-plasma concentration ratio is 1.3, suggesting complete brain penetration. However, the authors go on to note that the free brain-to-plasma concentration ratio in that study is 0.1.28 This free brain-to-plasma concentration ratio of less than 1 is to be expected as abemaciclib is a substrate of P-glycoprotein (P-gp), a transporter highly expressed at the BBB that limits the penetration of its substrates into the CNS. When considering a free brain-to-plasma concentration ratio of 0.1 for any molecule, the plasma concentration required to achieve benefit in the CNS behind the BBB is 10-fold higher than the target concentration to achieve benefit in peripheral tumors. Clearly, a difference in target concentration of 10-fold can impact whether therapeutic hypotheses can be tested, particularly with small-molecule kinase inhibitors, which tend to have narrow safety margins. The abemaciclib example reported by Eli Lily scientists serves as an excellent illustration of what is commonly encountered in small-molecule kinase inhibitor discovery programs.
Fig. 2.
Comparison of total and free brain-to-plasma concentration ratios in rat for abemaciclib.28
The above example demonstrates how the use of total brain-to-plasma concentration ratios can lead to misplaced optimism about the potential utility of a drug in the treatment of CNS malignancy. Without achieving adequate free drug concentration in the brain, conclusions about the merits of a therapeutic hypothesis should not be drawn. Fortunately, to assess molecules’ worth for study in the CNS oncology space, free brain penetration can be measured in preclinical studies by using a combination of measured total brain concentrations (ideally at numerous time points) along with assessment of brain tissue binding. Furthermore, the likelihood that a molecule will be able to achieve high free brain penetration can be determined in vitro using cell permeability assays that assess whether or not a molecule is a substrate of P-gp or breast cancer resistance protein (Bcrp). These are 2 of the primary transporters that limit small-molecule penetration of the BBB. If a molecule is a substrate of either of those transporters, free brain penetration will most likely be limited. If a molecule is not a substrate of those transporters, then there is a potential for the molecule to achieve free brain penetration, although it may still be limited by other factors, including inadequate permeability or the action of other efflux transporters. An illustrative example is the comparison of alectinib and crizotinib. Crizotinib is a reported substrate of P-gp and in a single case was reported to achieve a ratio of CSF to free plasma of 0.03.29,30 In contrast, alectinib is not a P-gp substrate and in a set of patients achieves near equivalent concentrations in CSF and free plasma.31,32
With an appreciation of the free drug hypothesis and that molecules that are substrates of P-gp or Bcrp will have limited ability to penetrate the BBB, the author assessed publicly available data for small-molecule inhibitors of kinases of interest in the brain cancer setting that have advanced to clinical trials.3 Data for the inhibitors were reviewed to determine if free brain penetration was measured/calculated (as opposed to total drug brain penetration), whether or not the molecule was determined to be a substrate of P-gp or Bcrp (limiting free brain penetration) or whether a pharmacodynamic effect had been demonstrated in brain tissue (conclusively demonstrating target engagement and, therefore, free brain penetration).
For the significant majority of small-molecule kinase inhibitors of targets of interest for CNS cancers that have advanced to clinical trials, including those studied in trials enrolling patients with brain cancers, there is insufficient evidence to expect high free drug penetration of the BBB (Table 1).3 Most of the kinase inhibitors reviewed that have advanced to clinical trials are substrates of P-gp, hence expected to have limited brain penetration. For many, in vivo preclinical studies have clearly demonstrated that brain penetration is limited by the action of P-gp. For another group of the kinase inhibitors reviewed, inadequate data exist to determine whether or not it is reasonable to expect meaningful free brain penetration. Of course, the absence of data does not allow for a conclusion that a molecule is a P-gp substrate and has limited potential for CNS penetration. However, in this author’s experience with kinase inhibitors, those that are not P-gp substrates are the exception rather than the rule, and the burden of proof should fall on the need to demonstrate free brain penetration rather than to expect it.
Table 1.
Summary of kinase targets of interest for neuro-oncology grouped according to whether known CNS penetrant clinical inhibitors are available (see Heffron3 for details)
| Kinase Targets with CNS Penetrant Clinical Inhibitors Available | Kinase Targets without Known CNS Penetrant Clinical Inhibitors |
|---|---|
| EGFR | VEGFR |
| PI3K/mTOR | AKT |
| ALK | IGF-1R |
| HER2 | CDK1/2 |
| MEK | b-RAF |
| Abl/Src | PLK1 |
| BTK | Aurora |
| PKC | |
| c-MET | |
| FAK/Pyk2 | |
| TGFR-b | |
| PIM1 | |
| ATM | |
| Mer | |
| AXL | |
| FGFR | |
| CDK4/6 |
There are many reasons why small molecules might fail in clinical studies. An inadequate pharmacokinetic profile may limit exposure. Toxicity might prevent achievement of drug exposure needed for efficacy. For the treatment of brain cancer, however, whether or not sufficient free drug concentration in the brain to provide efficacy can be realized is an additional consideration. So, while many kinase inhibitors have failed in clinical trials for the treatment of brain cancers, if those molecules were not capable of free penetration of the BBB, the biological hypotheses likely were not tested. That is, even if a drug can provide benefit in the treatment of peripheral tumors, it may not have potential to be an effective treatment for brain cancers simply because it might be restricted from achieving adequate concentrations in the CNS. In this scenario, it does not mean that the target of that inhibitor is not a valid therapeutic target in the treatment of CNS malignancy. Rather, to fully evaluate the therapeutic hypothesis, freely BBB-penetrating inhibitors are needed that also have the pharmacokinetic and safety profiles to enable meaningful study. Furthermore, as most cancers require combination treatment, in the neuro-oncology setting there may be the need for multiple brain penetrant partners in order to derive a benefit.
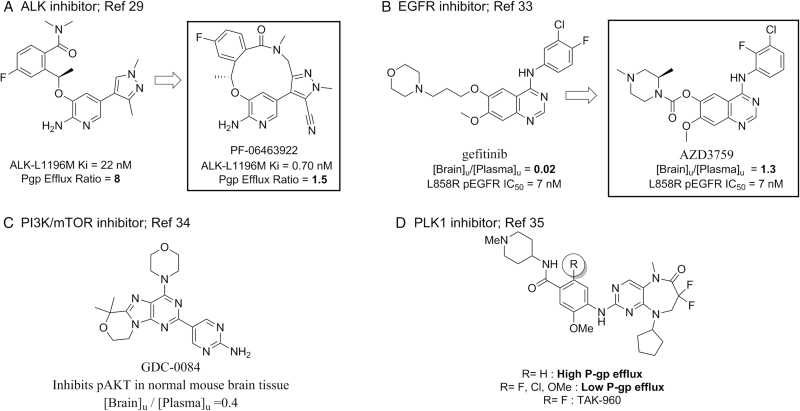
Encouragingly, in recent years there have been several small-molecule kinase inhibitors for brain cancers identified as capable of achieving meaningful free brain penetration.29,33,34 For these molecules, free brain penetration was assessed either through measurement of free drug levels in the brain or by demonstrating a pharmacodynamic effect in the CNS (Figure 3A–C). Another recent report describes kinase inhibitors designed specifically to avoid transporter efflux at the BBB, with the goal to achieve high free drug penetration in the brain (Figure 3D).35 Each of these examples highlights that the drug discovery field is beginning to recognize the importance of achieving free brain penetration to have an increased chance at effectively treating CNS cancers. Additionally, these examples demonstrate that physicochemical property optimization of kinase inhibitors during the discovery phase can lead to drug candidates that are potent and selective and have desirable pharmacokinetic properties that include free brain penetration. That is, the types of molecules required to enable clinical evaluation of hypotheses for the treatment of brain cancer can be realized.
Fig. 3.
Examples of kinase inhibitors for neuro-oncology designed and demonstrated to achieve high free brain penetration in preclinical studies (A–C) or designed and demonstrated to not be a substrate of P-gp (D). [Brain]u/[Plasma]u refers to the ratio of unbound, or free, brain and plasma concentrations in rodent pharmacokinetic studies.
By acknowledging the need for penetration of free drug across the BBB to treat CNS malignancy, drug discovery programs have the potential to design effective drug candidates that can evaluate therapeutic hypotheses. New, freely BBB-penetrating kinase inhibitors are needed to adequately assess the value of inhibiting particular kinases for the treatment of CNS cancers. Prior to advancement of new molecules to study for the treatment of brain cancers, candidates should be assessed for their ability to freely penetrate the BBB. Doing so should result in more effective clinical investigations and greater benefit to patients.
Conflict of interest statement. Timothy Heffron is an employee of Genentech, Inc and a shareholder of Roche.
References
- 1. Ostrom QT, Gittleman H, Xu J et al. . CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009–2013. Neuro Oncol. 2016;18(suppl 5):1–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Patchell RA. The management of brain metastases. Cancer Treat Rev. 2003;29(6):533–540. [DOI] [PubMed] [Google Scholar]
- 3. Heffron TP. Small molecule kinase inhibitors for brain cancer. J Med Chem. 2016;59(22):10030–10066. [DOI] [PubMed] [Google Scholar]
- 4. Bridges AJ. Chemical inhibitors of protein kinases. Chem Rev. 2001;101(8):2541–2572. [DOI] [PubMed] [Google Scholar]
- 5. Wu P, Nielsen TE, Clausen MH. FDA-approved small-molecule kinase inhibitors. Trends Pharmacol Sci. 2015;36(7):422–439. [DOI] [PubMed] [Google Scholar]
- 6. For inhibitors approved in 2015: US Food & Drug Administration. (2016) New Drug Approvals for 2015 https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DrugInnovation/ucm430302.htm. Accessed May 22, 2017.
- 7. For inhibitor approved in 2016: US Food & Drug Administration. (2017) New Drug Approvals for 2016 https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DrugInnovation/ucm483775.htm. Accessed May 22, 2017.
- 8. National Cancer Institute. (2010) FDA approval for everolimus https://www.cancer.gov/about-cancer/treatment/drugs/fda-everolimus#Anchor-SEGA. Accessed July 20, 2017.
- 9. US Food & Drug Administration. (2015) FDA approves new oral therapy to treat ALK-positive lung cancer https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm476926.htm. Accessed June 22, 2017.
- 10. Steeg PS, Camphausen KA, Smith QR. Brain metastases as preventive and therapeutic targets. Nat Rev Cancer. 2011;11(5):352–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sledge GW., Jr Heading in a new direction: drug permeability in breast cancer brain metastasis. Clin Cancer Res. 2010;16(23):5605–5607. [DOI] [PubMed] [Google Scholar]
- 12. Lockman PR, Mittapalli RK, Taskar KS et al. . Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res. 2010;16(23): 5664–5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Henson JW, Cordon-Cardo C, Posner JB. P-glycoprotein expression in brain tumors. J Neurooncol. 1992;14(1):37–43. [DOI] [PubMed] [Google Scholar]
- 14. Demeule M, Shedid D, Beaulieu E et al. . Expression of multidrug-resistance P-glycoprotein (MDR1) in human brain tumors. Int J Cancer. 2001;93(1):62–66. [DOI] [PubMed] [Google Scholar]
- 15. Claes A, Idema AJ, Wesseling P. Diffuse glioma growth: a guerilla war. Acta Neuropathol. 2007;114(5):443–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Agarwal S, Sane R, Oberoi R, Ohlfest JR, Elmquist WF. Delivery of molecularly targeted therapy to malignant glioma, a disease of the whole brain. Expert Rev Mol Med. 2011;13:e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kuratsu J, Itoyama Y, Uemura S, Ushio Y. Regrowth patterns of glioma—cases of glioma regrew away from the original tumor. Gan No Rinsho. 1989;35(11):1255–1260. [PubMed] [Google Scholar]
- 18. Silbergeld DL, Chicoine MR. Isolation and characterization of human malignant glioma cells from histologically normal brain. J Neurosurg. 1997;86(3):525–531. [DOI] [PubMed] [Google Scholar]
- 19. Lucio-Eterovic AK, Piao Y, de Groot JF. Mediators of glioblastoma resistance and invasion during antivascular endothelial growth factor therapy. Clin Cancer Res. 2009;15(14):4589–4599. [DOI] [PubMed] [Google Scholar]
- 20. Lin NU, Amiri-Kordestani L, Palmieri D, Liewehr DJ, Steeg PS. CNS metastases in breast cancer: old challenge, new frontiers. Clin Cancer Res. 2013;19(23):6404–6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Morikawa A, Peereboom DM, Smith QR et al. . Clinical evidence for drug penetration of capecitabine and lapatinib uptake in resected brain metastases from women with metastatic breast cancer. J Clin Oncol. 2013;31(suppl 15):Abstr. 514. [Google Scholar]
- 22. Swain SM, Baselga J, Miles D et al. . Incidence of central nervous system metastases in patients with HER2-positive metastatic breast cancer treated with pertuzumab, trastuzumab, and docetaxel: results from the randomized phase III study CLEOPATRA. Ann Oncol. 2014;25(6):1116–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Camidge DR, Bang YJ, Kwak EL et al. . Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012;13(10):1011–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Otterson GA, Riely GJ, Shaw AT et al. . Clinical characteristics of ALK+ NSCLC patients treated with crizotnib beyond disease progression: potential implications for management. J Clin Oncol. 2012;30(suppl 15):Abstr. 7600. [Google Scholar]
- 25. Smith DA, Di L, Kerns EH. The effect of plasma protein binding on in vivo efficacy: misconceptions in drug discovery. Nat Rev Drug Discov. 2010;9(12):929–939. [DOI] [PubMed] [Google Scholar]
- 26. Rankovic Z. CNS drug design: balancing physicochemical properties for optimal brain exposure. J Med Chem. 2015;58(6):2584–2608. [DOI] [PubMed] [Google Scholar]
- 27. Levin VA, Fenstermacher JD, Patlak CS. Sucrose and inulin space measurements of cerebral cortex in four mammalian species. Am J Physiol. 1970;219(5):1528–1533. [DOI] [PubMed] [Google Scholar]
- 28. Raub TJ, Wishart GN, Kulanthaivel P et al. . Brain exposure of two selective dual CDK4 and CDK6 inhibitors and the antitumor activity of CDK4 and CDK6 inhibition in combination with temozolomide in an intracranial glioblastoma xenograft. Drug Metab Dispos. 2015;43(9):1360–1371. [DOI] [PubMed] [Google Scholar]
- 29. Johnson TW, Richardson PF, Bailey S et al. . Discovery of (10R)-7-amino-12-fluoro-2,10,16-trimethyl-15-oxo-10,15,16,17-tetrahydro-2H-8,4-(metheno)pyrazolo[4,3-h][2,5,11]-benzoxadiazacyclotetradecine-3-carbonitrile (PF-06463922), a macrocyclic inhibitor of anaplastic lymphoma kinase (ALK) and c-ros oncogene 1 (ROS1) with preclinical brain exposure and broad-spectrum potency against ALK-resistant mutations. J Med Chem. 2014;57(11):4720–4744. [DOI] [PubMed] [Google Scholar]
- 30. Costa DB, Kobayashi S, Pandya SS et al. . CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol. 2011;29(15):e443–e445. [DOI] [PubMed] [Google Scholar]
- 31. Kodama T, Hasegawa M, Takanashi K, Sakurai Y, Kondoh O, Sakamoto H. Antitumor activity of the selective ALK inhibitor alectinib in models of intracranial metastases. Cancer Chemother Pharmacol. 2014;74(5):1023–1028. [DOI] [PubMed] [Google Scholar]
- 32. Gadgeel SM, Gandhi L, Riely GJ et al. . Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase ½ study. Lancet Oncol. 2014;15(10):1119–1128. [DOI] [PubMed] [Google Scholar]
- 33. Zeng Q, Wang J, Cheng Z et al. . Discovery and evaluation of clinical candidate AZD3759, a potent, oral active, central nervous system-penetrant, epidermal growth factor receptor tyrosine kinase inhibitor. J Med Chem. 2015;58(20):8200–8215. [DOI] [PubMed] [Google Scholar]
- 34. Heffron TP, Ndubaku CO, Salphati L et al. . Discovery of clinical development candidate GDC-0084, a brain penetrant inhibitor of PI3K and mTOR. ACS Med Chem Lett. 2016;7(4):351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nie Z, Feher V, Natala S et al. . Discovery of TAK-960: an orally available small molecule inhibitor of polo-like kinase 1 (PLK1). Bioorg Med Chem Lett. 2013;23(12):3662–3666. [DOI] [PubMed] [Google Scholar]