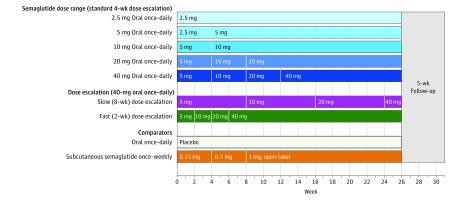
Figure 1. Trial Design.
The trial was conducted among 632 patients with type 2 diabetes who were 18 years or older receiving stable treatment with diet and exercise or stable treatment with metformin for 30 days prior to screening. Patients had a hemoglobin A1c level of 7.0% to 9.5% and an estimated glomerular filtration rate of 60 mL/min/1.73 m2 or more. There was no dose escalation in the oral semaglutide 2.5-mg and placebo groups. In the other oral semaglutide groups, the dose was doubled from a starting dose of 2.5 mg or 5 mg every 4 weeks until the trial maintenance dose of the group (5-40 mg) was achieved (blue shades). The subcutaneous semaglutide dose was doubled every 4 weeks from a starting dose of 0.25 mg until a 1 mg trial maintenance dose was achieved. In addition, a slow dose escalation (purple) to 40 mg of oral semaglutide at 8-week intervals and a fast dose escalation (green) to 40 mg of oral semaglutide at 2-week intervals were included. Subcutaneous semaglutide was supplied as a 1.34-mg/mL solution in a 1.5-mL prefilled PDS290 pen injector (FlexTouch, Novo Nordisk A/S), and was administered in the abdomen, thigh, or upper arm on the same day of the week.