ABSTRACT
Mammalian mastication involves precise jaw movements including transverse movement of the mandible during the power stroke. Jaw elevation and transverse movement are driven by asymmetrical jaw elevator muscle activity, which is thought to include a phylogenetically primitive and conserved triplet motor pattern consisting of: triplet I (balancing side: superficial masseter and medial pterygoid; working side: posterior temporalis), which reaches onset, peak and offset first; and triplet II (working side: superficial masseter and medial pterygoid; balancing side: posterior temporalis), which is active second. Although the presence of a triplet motor pattern has been confirmed in several primate species, the prevalence of this motor pattern – i.e. the proportion of masticatory cycles that display it – has not been evaluated in primates. The present study quantifies the presence and prevalence of the triplet motor pattern in five different primate species, Eulemur fulvus, Propithecus verreauxi, Papio anubis, Macaca fuscata and Pan troglodytes, using mean onset, peak and offset time relative to working superficial masseter. In all five of the species studied, the mean triplet motor pattern was observed at peak muscle activation, and in four out of the five species the triplet motor pattern occurred more frequently than expected at random at peak muscle activation and offset. Non-triplet motor patterns were observed in varying proportions at different time points in the masticatory cycle, suggesting that the presence or absence of the triplet motor pattern is not a binomial trait. Instead, the primate masticatory motor pattern is malleable within individual cycles, within individual animals and therefore within species.
KEY WORDS: Motor pattern, Motor synergy, Chewing, EMG
Summary: The prevalence of the jaw elevator triplet motor pattern during mastication is established in five different primate species.
INTRODUCTION
Motor patterns are cyclic or ‘repeating sequence(s) of motor neuron activity produced during an actual or fictive motor act’ (Binder et al., 2009). Cyclic chewing behavior is characterized primitively – and in many extant mammals – by precise occlusion, unilateral chewing, high levels of rhythmicity (Ross et al., 2010) and lateral-to-medial tooth and jaw movements on the biting side during the slow close phase (power stroke) of the jaw gape cycle (Hiiemae, 1976; Williams et al., 2011). The transverse movements during slow close are often ascribed to a specific motor pattern characterized by asymmetric activation (both in amplitude and timing) of the bilateral jaw elevator muscles – masseters, temporales and medial pterygoids (Herring and Scapino, 1973; Herring, 1976; Herring et al., 1979; Gorniak, 1977, 1985; Weijs and Dantuma, 1980). Weijs (1994) reified the triplet motor pattern into an ancestral motor pattern modified by natural selection to produce the range of motor patterns observed in extant mammals (shown in Fig. 1).
Fig. 1.
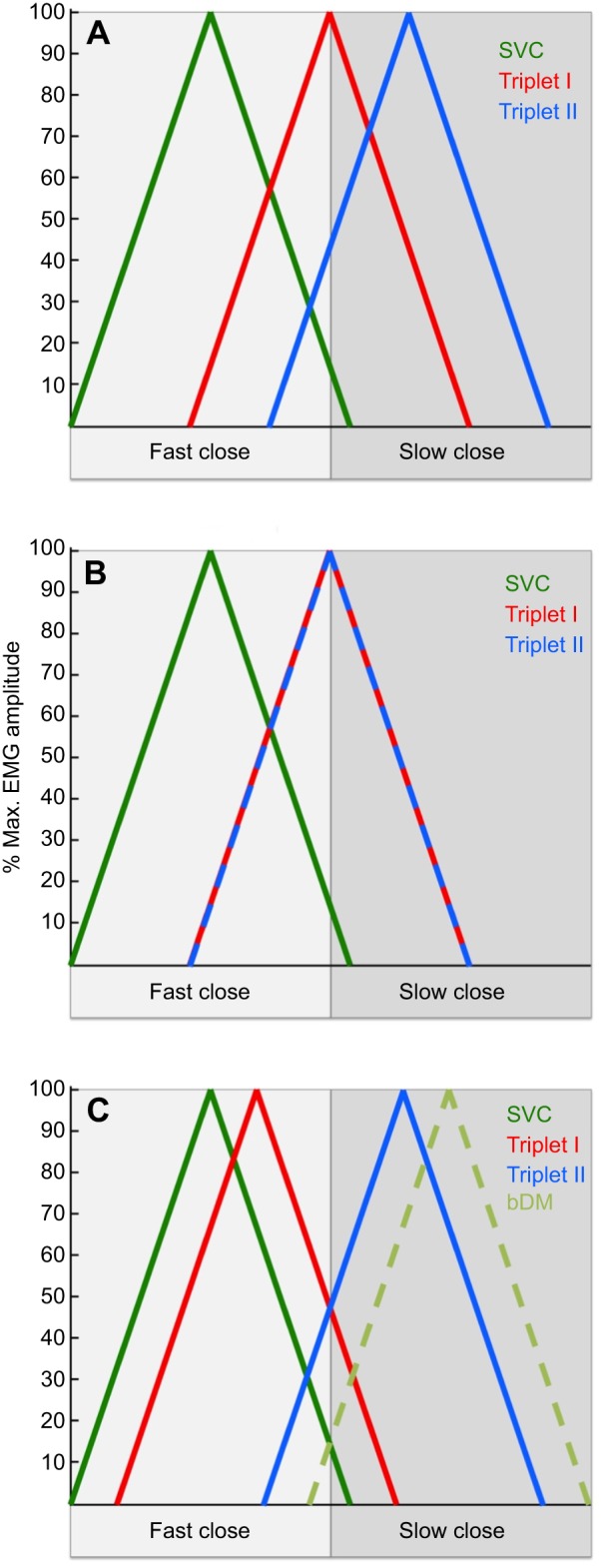
Illustration of Weijs's (1994) hypothesis that the primitive jaw adduction motor pattern is modified in different clades of extant mammals. The symmetric vertical closers (SVCs) include balancing- and working-side deep masseters (bDM and wDM) and anterior temporalis (bAT and wAT). (A) the primitive motor pattern; (B) the carnivore motor pattern; (C) the transverse motor pattern.
According to the triplet hypothesis, the lateral–medial working- side jaw movements during jaw elevation are produced by a specific activation sequence of the working-side (w) and balancing-side (b) superficial masseters (SM), posterior temporales (PT), and medial pterygoids (MP). In Weijs' primitive motor pattern, the symmetric vertical closers (SVCs) – balancing- and working-side deep masseters (bDM and wDM) and anterior temporalis (bAT and wAT) – fire first during the chewing cycle, followed by triplet I (bSM, bMP and wPT), which rotates the mandible towards the working side as it is elevated, then triplet II (wSM, wMP and bPT), which rotates the jaw towards the balancing side at the end of jaw elevation.
Weijs (1994) hypothesized that this primitive mammalian masticatory motor pattern has been modified differently in different groups of extant mammals (Fig. 1). He suggested that in carnivores, the two triplets are active at the same time, producing a predominantly vertical jaw motion which, in combination with the carnivore dental morphology, produces the vertical occlusal shearing movement appropriate for a carnivorous diet. In contrast, ungulates and large herbivores increase the temporal offset between triplets I and II in order to increase transverse movement of the jaw, producing the grinding movement associated with a herbivorous diet. Weijs (1994) hypothesized that in species with a transverse motor pattern, either the SVCs fire as a group before both triplet I and II, or the bDM fires after triplet II (as in many anthropoid primate species).
List of abbreviations.
- AT
anterior temporalis
- b
prefix indicating balancing side
- DM
deep masseter
- M
masseter
- MP
medial pterygoids
- PT
posterior temporalis
- RMS
root mean square
- SM
superficial masseters
- SVCs
symmetric vertical closers
- T
temporalis
- w
prefix indicating working side
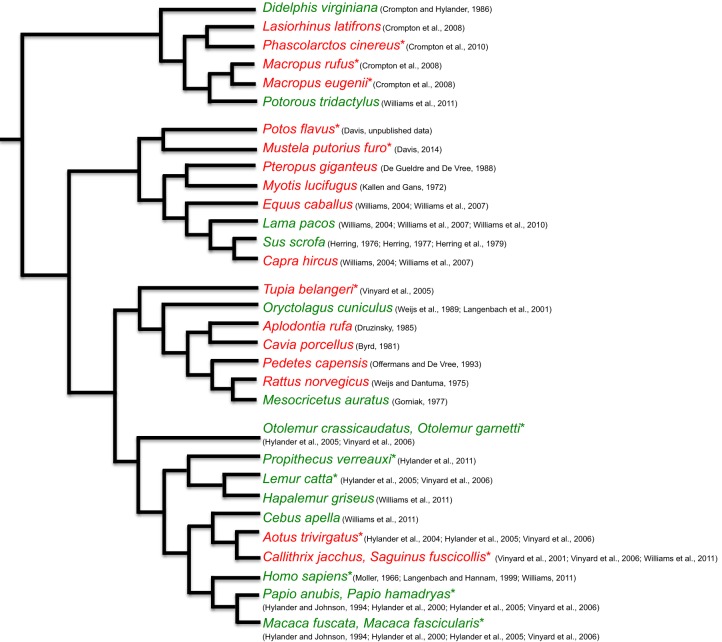
Support for the triplet hypothesis is variable and clade specific (Fig. 2, Table 1). In macropod marsupials, an orthal (vertical) phase of jaw closing is accompanied by activity in the bSM and bMP, closely followed by working- and balancing-side AT and PT (Crompton et al., 2008a), then a transverse jaw movement phase is accompanied by activity of wSM and wMP (Crompton et al., 2008a). In wombats, triplet motor patterns are not seen, as only the working-side jaw elevators are recruited during a completely transverse jaw-closing phase (Crompton et al., 2008b). Among macropod marsupials, only koalas display a triplet motor pattern: the wDM reaches a peak first, followed closely by the wAT in association with triplet I, the bAT in association with triplet II, followed by the bDM. In tree shrews (Tupaia), small, insectivorous, frugivorous mammals often argued to be the primate sister group, triplets I and II are observed but the SVCs are not all active before triplet I (Vinyard et al., 2005). Instead, the ATs are active with their ipsilateral PTs, the bDM fires in association with triplet I, and the wDM fires in association with triplet II. Williams et al. (2007) found little support for the triplet jaw elevator motor pattern in ungulates. In alpacas, the number of experiments that followed the triplet motor pattern was significant (based on a one-tailed binomial test) at onset but not at peak or offset, whereas in goats and horses, the triplet motor pattern was insignificant at onset, peak and offset. Thus, the marsupial and ungulate jaw elevator motor patterns provide little support for Weijs’ (1994) model of triplet motor pattern evolution: a triplet motor pattern is only observed in one marsupial and (partially) one ungulate.
Fig. 2.
The phylogeny of species where the triplet motor pattern either has or has not been supported. The phylogeny is based on Meredith et al. (2011) and Perelman et al. (2011). Green indicates species where the triplet motor pattern has been corroborated. Red indicates species where the triplet motor pattern has not been supported. Asterisks indicate that mean peak times relative to the working-side superficial masseter (wSM) are also provided. The branch lengths are arbitrary.
Table 1.
Peak muscle firing time (mean±s.d.) relative to working-side superficial masseters (wSM)
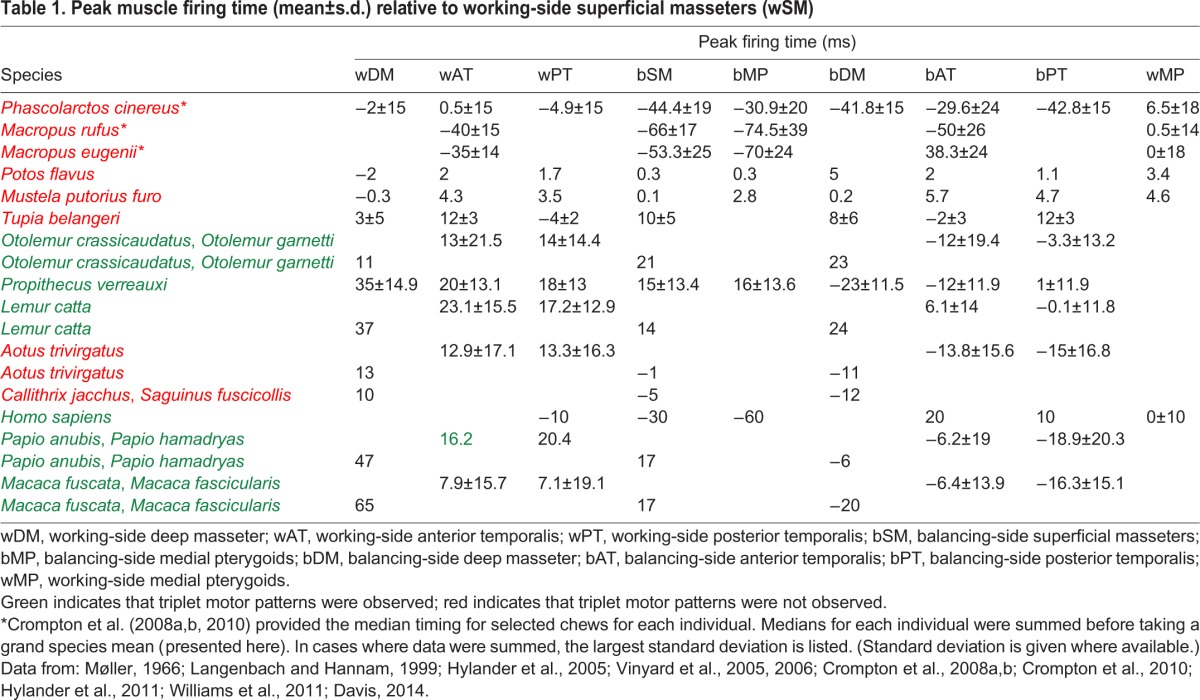
In primates, the triplet motor pattern has been identified in Sapajus and Cebus (Williams et al., 2011), Homo (Møller, 1966; Langenbach and Hannam, 1999), Papio and Macaca, but not in Aotus and Callithrix (Hylander and Johnson, 1994; Hylander et al., 2000, 2005; Vinyard et al., 2006). Hylander and colleagues relate variation in the triplet motor pattern to variation in jaw morphology and kinematics (Hylander and Johnson, 1985, 1994; Hylander et al., 1987, 2000, 2004, 2005). Strepsirrhines, the sister group to tarsiers and anthropoid primates, mostly have unfused mandibular symphyses, which Weijs (1994) predicted would be associated with the primitive mammalian motor pattern, and anthropoids (New and Old World monkeys) have fused symphyses, which Weijs (1994) predicted would be associated with the transverse motor pattern characteristic of other herbivorous mammals (Hylander, 1984; Hylander and Johnson, 1985). In galagos (Otolemur), the triplet motor pattern is observed, but the bDM fires with triplet I and the wDM fires with triplet II: the SVCs do not fire before triplet I. Hylander et al. (2000) noted that delayed and increased bDM activity in anthropoids prolongs the power stroke and increases transverse jaw movements, suggesting that late recruitment of the bDM and lower working/balancing DM ratios in anthropoids increase transverse components of bite force and contribute to the wish-boning deformation regime of the mandible during the power stroke. They hypothesized that this might be related to the evolution of symphyseal fusion in anthropoid primates, a hypothesis corroborated by the masticatory motor patterns of Propithecus verreauxi, a strepsirrhine that gradually develops a partially fused mandibular symphysis after birth, displaying nearly complete symphyseal fusion by adulthood (Hylander et al., 2011). Because Propithecus evolved symphyseal fusion independently of anthropoids, Hylander et al. (2011) predicted that they would also demonstrate anthropoid-like jaw elevator motor patterns. As predicted, Propithecus display increased bDM and bPT muscle activity amplitudes, and firing of DM with the contralateral SM, not the SVCs (Hylander et al., 2011). The lineage leading to extant Propithecus also evolved – convergently with anthropoid primates – a fused mandibular symphysis, condyles positioned high above the tooth row and vertically aligned jaw muscles, supporting the hypothesis that modified motor patterns are correlated with modified morphology and kinematics in primates (Ravosa et al., 2000).
Together, these data suggest that there is interspecific variation in the existence of the triplet motor pattern, and that the occurrence of a triplet motor pattern may be related to other aspects of feeding system structure and function (Fig. 2). Williams et al. (2011) tested for the concerted evolution of triplet I and II using pre-existing data, treating triplet I and II as binomial traits (i.e. present or absent) and using maximum likelihood and the Bayesian Markov chain Monte Carlo method to test for correlated evolution across the mammalian phylogeny. Their hypothesis was corroborated by both tests, suggesting that the two triplets evolve together and hence may be acted on by natural selection for their advantage according to some unspecified optimality criterion.
The literature reviewed above argues for ‘consistently identifiable patterns of muscular contraction that characterize feeding behaviors both across individuals and among species. These muscle activity patterns, or motor patterns, are characterized by consistent order, duration and/or magnitude of muscle activation during specific feeding tasks’ (Williams et al., 2011, p.248). In addition to this inter-specific variation in the existence of triplet motor patterns, there is also variability in the prevalence of the motor pattern between cycles within individual animals that is rarely quantified but important for understanding the control of mammalian jaw movements. To date, the only paper documenting this variability is Williams et al. (2007) in which a one-tailed binomial test was used to evaluate the hypothesis that the triplet motor pattern is the most common motor pattern at the onset of activity, peak activity and offset of activity. However, by first calculating the experimental mean onset, peak and offset times, then testing the hypothesis using the experimental means, this method de-emphasizes intra-individual variation within experiments and takes into consideration only the inter-individual variation within a species.
The importance of cycle-to-cycle variation in relative muscle timing is highlighted by studies showing that there is more variation in jaw elevator muscle relative timing and jaw kinematics within chewing sequences on a single piece of food than between chewing sequences on different foods (Vinyard et al., 2008; Ross et al., 2012; Ross and Iriarte-Diaz, 2014). With this in mind, the present study documents variability in the triplet motor pattern across primates. The existence of a triplet motor pattern in primate species is suggested by the mean timing of peak muscle activity relative to wSM (Hylander et al., 2000, 2005; Vinyard et al., 2005), i.e. all the muscles in triplet I (bSM, bMP, wPT) reach peak muscle activity before all the muscles in triplet II (wSM, wMP, bPT). However, the evolutionary and functional significance of the triplet motor pattern is also related to the proportion of chewing cycles displaying a triplet motor pattern at onset, peak and offset. Data on the proportion of cycles that follow the triplet motor pattern in each species promise insight into the distribution and variability in jaw elevator motor patterns within primate clades. Moreover, by looking at three different time points during the gape cycle (onset of muscle activity, peak muscle activity and offset of muscle activity), this study sheds light on the biomechanical factors influencing the prevalence of the triplet motor pattern, informing hypotheses about the selection pressures establishing and maintaining triplet motor patterns in primate lineages. In sum, understanding when and how frequently the triplet motor pattern occurs will be helpful in understanding not only whether the triplet motor pattern is more common than other jaw elevator motor patterns but also why the triplet motor pattern is so common in the mammalian clade.
Hence, this research asks two questions: (1) what proportion of chewing cycles displays a triplet motor pattern?; and (2) do the jaw elevator muscles show consistent patterns of ‘triplet’ behavior at onset, peak and offset?
MATERIALS AND METHODS
Data selection
Data used in this study are from adult Propithecus verreauxi A. Grandidier 1867, Papio anubis (Lesson 1827) and Macaca fuscata Raffles 1821, which were downloaded from the FEED database (Wall et al., 2011), and from adult Eulemur fulvus É. Geoffroy 1796 and Pan troglodytes (Blumenbach 1776), which were extracted from data files previously collected by one of us (C.F.R.). The number of chewing cycles used per species is denoted in Table 2. Sequences were selected for analysis if the chewing side could be identified and the EMG data included enough triplet muscles and were of good quality (not clipped or too noisy). Sequences from the FEED database that had EMG values for bSM, wSM, bPT and wPT were utilized in this study. If chewing sequences in the database included two channels of recordings from the same muscle, one of the two signals was arbitrarily chosen based on the following criteria: most constant baseline, least baseline noise and largest unclipped amplitude during rhythmic mastication.
Table 2.
Number (N) of masticatory cycles per individual
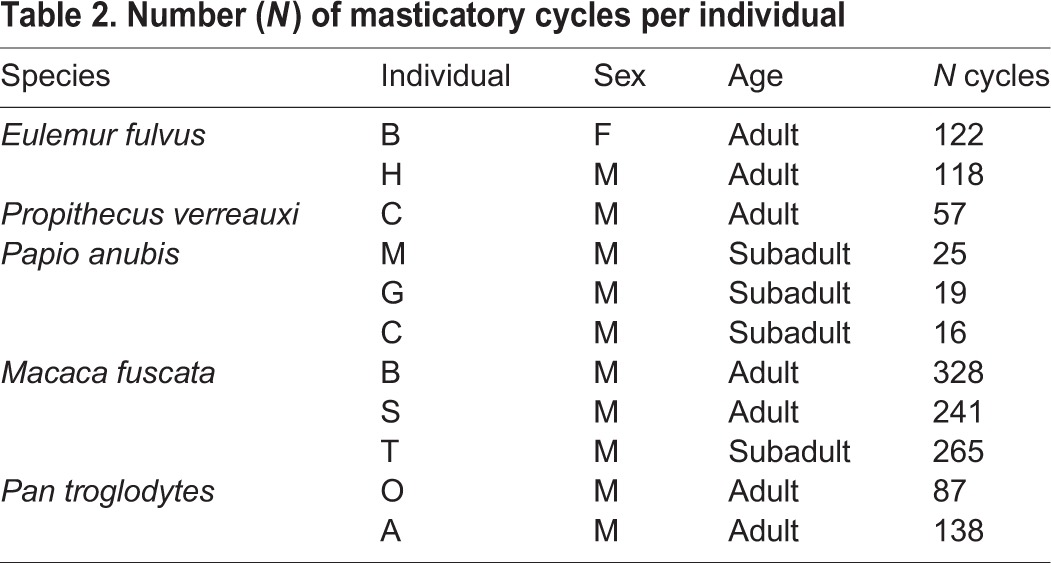
Chewing sequences for M. fuscata, P. anubis and P. verreauxi were labeled in the FEED database as exclusively left or right chews. Chewing side for E. fulvus was recorded on the voice track of the video or in experimental notes during data collection and corroborated using changes in principal strain orientation recorded from the mandible. For P. troglodytes, the chewing side was determined from the direction of jaw movement during the slow close phase of the gape cycle as seen on videos of the recording session: if the mandible was moving towards the left, then it was a right chew and vice versa. However, jaw movement was not visible for all cycles, so for the remaining cycles, a clustering algorithm that utilized EMG data for all jaw elevator muscles was used to determine the working side. The clustering algorithm successfully classified all those cycles for which the working side could be seen in the video and so it was assumed to accurately reconstruct chewing side for the remaining cycles. Data were collected as animals chewed various foods including but not limited to grapes, apples, carrots, dried papayas and cherries, prunes, raisins and popcorn. All the data were collected at 10 kHz with exception of P. troglodytes and E. fulvus data (1 kHz).
Pre-processing
The data were full-wave rectified and a 4th order low-pass Butterworth filter with cutoff at 30 Hz was applied followed by a root mean square (RMS) moving window integration with a 42 ms integration window. For each sequence, all four channels were plotted and the starting point for each cycle manually selected such that all four cycles had minimal EMG activity at the start of the cycle and each channel reached peak amplitude only once during the cycle (in theory, jaw elevation muscles are minimally active at maximum gape; thus, the cycle start and end approximate maximum gape). Every cycle was manually reviewed to ensure that no channels were clipped and all cycles represented only one complete cycle of jaw-closing EMGs. Muscle names were changed to include working side and balancing side. Individual channel amplitudes were normalized from 0 to 1 within each cycle by subtracting the minimum and dividing by the maximum value. Examination of the integrated and rectified EMG signal for the MP from the FEED database revealed EMG activity during jaw depression (Fig. 3). This was interpreted as either the electrode erroneously inserted into the digastric muscle or cross-talk between the medial pterygoid and the posterior belly of the digastric muscle: therefore, all medial pterygoid data were excluded from the analysis.
Fig. 3.
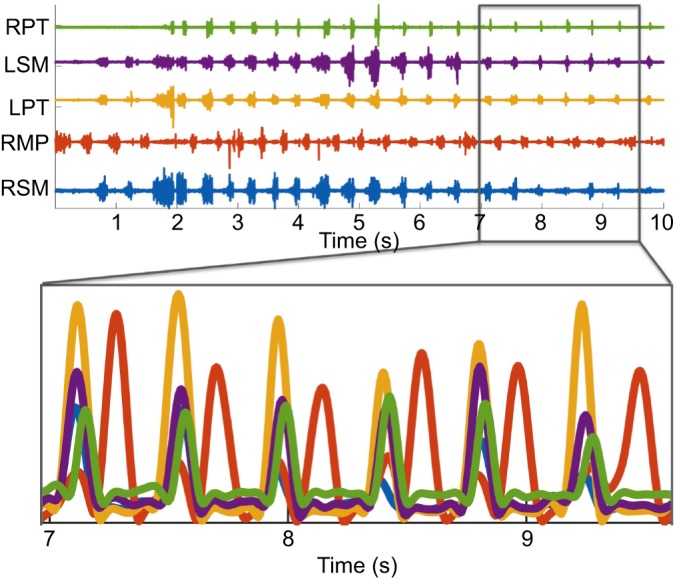
EMG of a feeding sequence from the FEED database (www.feedexp.org) for the medial pterygoids (MP). (A) The raw EMG data for each channel. (B) The EMG after it was filtered (see Materials and methods for details). Note that the red channel (RMP/ RMPT) is out of phase with all of the other channels. One bout is in phase with the jaw elevators and the other is anti-phase with the jaw elevators. This indicates that there is cross-talk between the MP and the digastric muscle. For this reason, MP was excluded from our study. RPT, right posterior temporalis; LSM, left superficial masseter; LPT, left posterior temporalis; RMP, right medial pterygoid; RSM, right superficial masseter.
Mean onset, peak and offset time
Onset was defined as the last time point before peak amplitude when standardized amplitude was ≤0.25. Peak amplitude was defined as the time point in the cycle when standardized amplitude equaled 1.0. Offset was defined as the first time point after peak amplitude when amplitude was ≤0.25. The offset could occur up to 8 ms after the manually selected end of the cycle (which approximates maximum gape). Onset, peak and offset frames were multiplied by recording frequency to obtain onset, peak and offset time. Finally, onset, peak and offset time of the wSM was subtracted from that of all other muscles to obtain onset, peak and offset times relative to wSM. Onset and peak times less than 30% of total cycle length were discarded and offset times less than or equal to 60% of total cycle length were discarded. If both onset and offset times for a given cycle were discarded, then the cycle was excluded from all further analyses. Cycles with a peak amplitude time less than onset or greater than offset were also excluded from all further analyses.
The standard technique for determining whether a species follows the triplet motor pattern involves calculating the mean muscle onset, peak and offset times (see Table 3 for a list of studies that have previously used this method). Based on this method, if all the muscles in triplet I have mean onset, peak and offset times before those of triplet II, then the species follows the triplet motor pattern. A one-way t-test was used to determine whether the distribution of onset, peak and offset times was significantly different (P≤0.05) from a normal distribution around zero (onset, peak and offset time for wSM) with unknown variance.
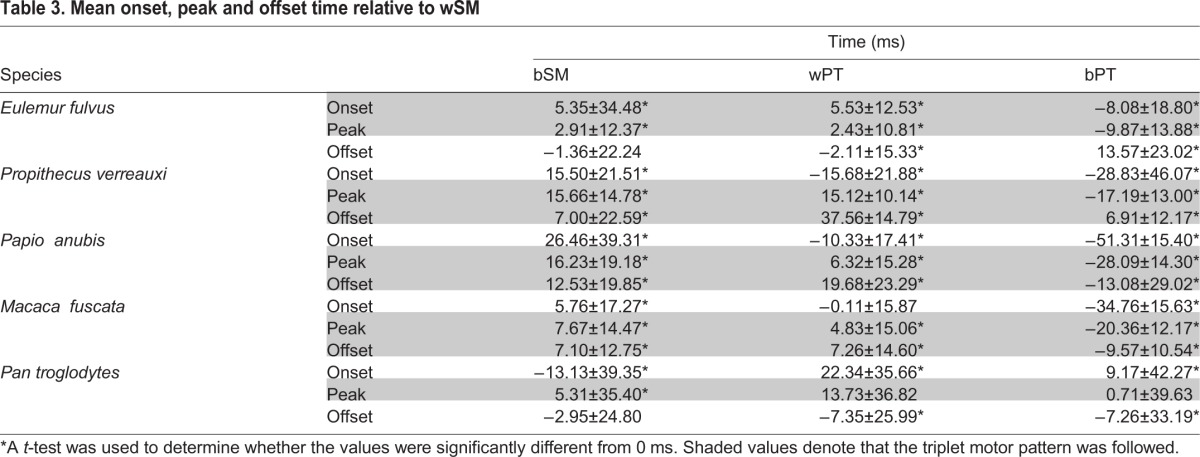
Table 3.
Mean onset, peak and offset time relative to wSM
Proportion of cycles that follow the triplet motor pattern
Only cycles with valid peaks for both SM and PT muscles were analyzed. Channels were ordered by peak time (earliest to latest). If the first two channels were bSM and wPT (in any order), the cycle followed the triplet motor pattern. This process was repeated for onset and peak, and for all valid cycles.
The probability that the first muscle will fit the triplet motor pattern is two out of four possible muscles. The probability that the second muscle will fit the triplet motor pattern is one out of three possible muscles. The product of these two fractions yields the probability that the cycle will follow the triplet motor pattern (1/6). One-tailed binomial probability tests were used to test whether the actual probabilities were significantly (P≤0.05) different from 16.66%.
Here, the percentage of cycles that follow the triplet motor pattern determines whether a particular species follows the triplet motor pattern more frequently than expected by random probability. Using the percentage of cycles that follow the triplet motor pattern as opposed to the mean ensures that the variability in the data is appropriately captured. Comparing this with random probability implies that if the triplet motor pattern occurs more frequently than expected at random then there must be a functional and/or physiological reason for it. For this portion of the study, if the percentage of cycles that follow the triplet motor pattern exceeds the percentage predicted by random probability at onset, peak and offset, then that species follows the triplet motor pattern.
Additionally, for each cycle, the order in which muscles reach onset, peak and offset was calculated. There are 24 total possible permutations of the four muscles. Based on random probability, each permutation will be observed 4.17% of the time. One-tailed binomial probability tests were used to test whether the actual probabilities were significantly (P≤0.05) different from 4.17%. Once again, by comparing this with random probability, we assume that if that particular permutation occurs more frequently than expected at random, then there must be a functional and/or physiological reason for it.
RESULTS
Mean onset, peak and offset time
Table 3 shows the mean onset, peak and offset times for the jaw elevators relative to the wSM for all species. Using these mean values of onset, peak and offset, E. fulvus follows the triplet motor pattern at onset and peak but not offset. Although the timing of bSM activity is more variable than that of wPT, the mean onset, peak and offset times for both muscles are separated by 0.75 ms or less. In E. fulvus, bPT is consistently the last muscle to reach onset, peak and offset.
Propithecus verreauxi follows the triplet motor pattern at peak and offset but not onset. Based on the mean onset time, wPT reaches onset before wSM. Although the triplet motor pattern is maintained during offset, wSM reaches offset after bPT. The mean offset times of bPT and bSM are separated by just 0.09 ms. Papio anubis follows the triplet motor pattern at peak and offset but not onset. All onset peak and offset times were significantly different from 0 ms in P. anubis.
Like P. verreauxi and P. anubis, M. fuscata also follows the triplet motor pattern at peak and offset but not at onset. The mean onset time for wPT is just −0.11±15.87 ms after that of wSM. Once again, like P. verreauxi and P. anubis, bPT reaches offset before wSM.
Pan troglodytes follows the triplet motor pattern at peak activity only. The mean onset times for wPT and bPT are earlier than the onset time of wSM and bSM. The distribution of peak activity time for bPT is not significantly different from that of wSM. Thus, the two muscles in triplet II may reach peak close to simultaneously.
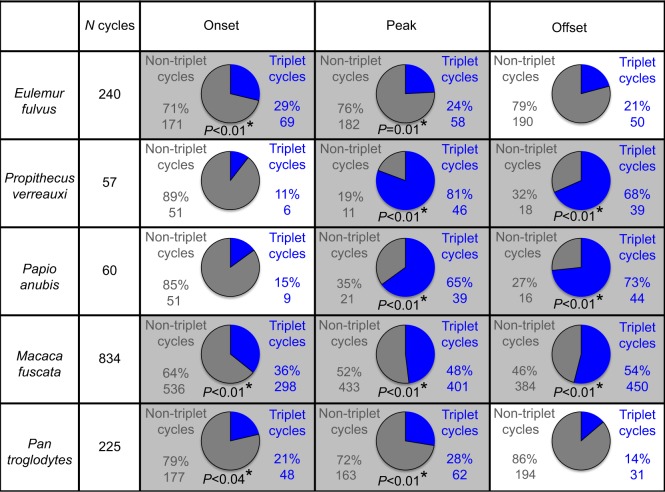
What percentage of cycles follows the triplet motor pattern?
The proportion of cycles that follow the triplet pattern at onset, peak and offset is illustrated in Fig. 4. A cycle follows the triplet motor pattern if all the channels in triplet I (bSM, wPT) reach peak, onset or offset before all the muscles in triplet II (wSM, bBT); i.e. for each cycle, the triplet motor pattern was assessed separately at onset, peak and offset.
Fig. 4.
The number (N) of masticatory cycles per species that follow the triplet motor pattern. Each species has three pie charts, one each for onset, peak and offset. Cycles that follow the triplet motor pattern are shown in blue while cycles that do not follow the triplet motor pattern are shown in gray. The number and percentage to the left of each pie chart represent the number and percentage of cycles that do not follow the triplet motor pattern. The number and percentage to the right of each pie chart represent the number and percentage of cycles that do follow the triplet motor pattern. If the number of cycles that follow the triplet motor pattern is significantly greater (*P≤0.05) than the number expected by random probability (16.66%), then the P-value is depicted below the pie chart and the box is shaded.
In E. fulvus, 29% of all cycles followed the triplet motor pattern at onset, while 24% of cycles followed the triplet motor pattern at peak and 21% of cycles followed the triplet pattern at offset. The proportions were significantly different from random at onset and peak but not at offset (P<0.01, P=0.01 and P=0.05, respectively). For E. fulvus, 17.92% of cycles followed the triplet motor pattern at both onset and peak while 12.92% of cycles followed the triplet motor pattern at both peak and offset. Only 8.33% of cycles followed the triplet motor pattern at onset, peak and offset.
The second strepsirrhine species studied, P. verreauxi, had the smallest sample size with 57 cycles. In P. verreauxi, 11% of cycles followed the triplet motor pattern at onset, 81% of cycles followed the triplet motor pattern at peak amplitude and 68% followed the triplet motor pattern at offset. Both peak and offset were significantly different from random probability (P<0.01 for both). Only 7.02% of cycles followed the triplet motor pattern at onset, peak and offset. For P. verreauxi, 10.53% the cycles followed the triplet motor pattern at both onset and peak while 57.89% of cycles followed the triplet motor pattern at both peak and offset. Hence, P. verreauxi followed the triplet motor pattern at peak muscle activation and at offset.
Among the anthropoids, M. fuscata followed the triplet motor pattern at onset (36% of cycles), peak (48%) and offset (54%) (P<0.01). Here, 29.02% of cycles followed the triplet motor pattern at both onset and peak while 37.53% of cycles followed the triplet motor pattern at both peak and offset. Only 22.06% of cycles followed the triplet motor pattern at onset, peak and offset. Papio anubis followed the triplet motor pattern at peak (65%, P<0.01) and offset (73%, P<0.01) but not at onset (15%). All 15% of cycles that followed the triplet motor pattern at onset also followed it at peak, while 46.67% of cycles that followed the triplet motor pattern at peak also followed the triplet motor pattern at offset. However, only 5% of cycles followed the triplet motor pattern at onset, peak and offset. Cycles are more likely to follow the triplet motor pattern at only one or two points in the cycle than to maintain the triplet motor pattern throughout the cycle.
Pan troglodytes, the only hominid studied, followed the triplet motor pattern at onset and peak but not at offset. Here, 21% of cycles followed the triplet motor pattern at onset, 28% followed the motor pattern at peak and 14% followed the triplet motor pattern at offset. In fact, at offset, the number of cycles that followed the triplet motor pattern was significantly less than that expected by random probability. Only 8% of cycles followed the triplet motor pattern at onset and peak, while only 5% of cycles followed the triplet motor pattern at peak and offset. Only 2.22% of cycles followed the triplet motor pattern at onset, peak and offset.
Specific motor patterns occurred more frequently than expected by random probability in all five species (Table 4). In both E. fulvus and M. fuscata, the same four permutations occurred more often than expected by random probability at onset, peak and offset. These four permutations were: (1) wPT, wSM, bSM, bPT; (2) wPT, bSM, wSM, bPT; (3) bSM, wSM, wPT, bPT; and (4) bSM, wPT, wSM, bPT. Permutations 2 and 4 follow the triplet motor pattern. Permutations 1 and 2 begin with wPT and end in bPT; permutation 2 follows the triplet motor pattern but in permutation 1, wSM fires before bSM. The mean temporal offset between the two muscles is 5.91±4.64 ms in E. fulvus and 5.36±5.00 ms in M. fuscata at peak amplitude. It is currently unclear how this temporal offset affects jaw kinematics. In the final sequence that occurs more frequently than expected by random probability at all three time points, the two SM reach onset, peak and offset before the two PT. Additionally, in E. fulvus, the permutation wPT, wSM, bPT, bSM occurs more frequently than expected by random probability at onset and offset but not at peak. In M. fuscata, the permutation wSM, bSM, bPT, wPT occurs more frequently than expected by random probability at onset and peak but not offset.
Table 4.
Permutations in muscle order at onset, peak and offset
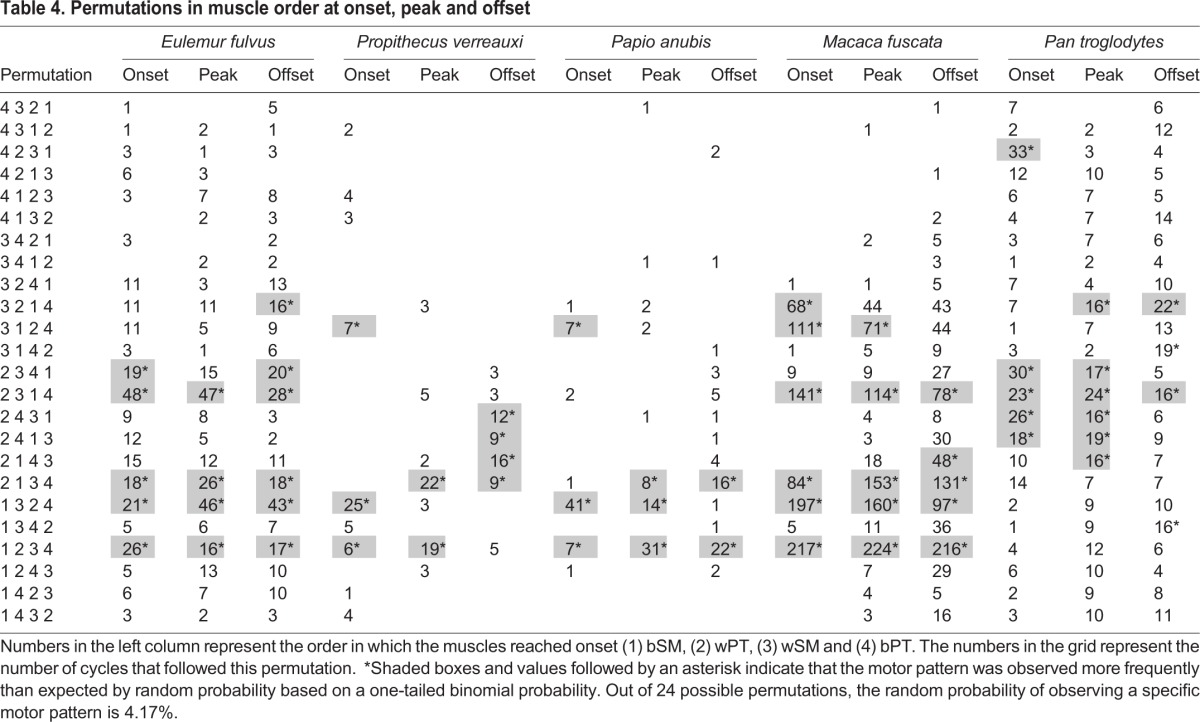
In P. verreauxi, no permutations were observed more frequently than expected by random probability at all three time points. However, two triplet permutations do occur more frequently than expected by random probability at two out of three time points. In P. anubis, just one triplet permutation occurs more frequently than expected by random probability at all three time points. One more triplet permutation occurs more frequently than expected by random probability at peak and offset but not at onset. Finally, the permutation bSM, wSM, wPT, bPT occurs more frequently than expected by random probability at onset and peak but not at offset. Pan troglodytes was unlike the other species because triplet permutations did not occur more frequently than expected by random probability at any of the time points. Instead, the permutation wSM, bSM, bPT, wPT occurs more frequently than expected by random probability at onset, peak and offset. Three additional permutations occur more frequently than expected by random probability at onset and peak but not offset. All three of these permutations start with wPT. In one sequence, the second muscle is wSM, while in the other two it is bPT. Finally, the permutation wSM, wPT, bSM, bPT occurs more frequently than expected by random probability at peak and offset in P. troglodytes but not at onset.
DISCUSSION
The purpose of this study was to quantify variability in jaw elevator motor patterns using two different metrics to determine which species follow the triplet motor pattern at three different times in the masticatory cycle: onset of muscle activity, peak muscle activity and offset of muscle activity. The hypotheses tested and whether they were corroborated is illustrated in Fig. 5. The species studied included Propithecus verreauxi, Papio anubis, Macaca fuscata, Eulemur fulvus and Pan troglodytes. Following previous studies, the mean onset, peak and offset times were calculated relative to wSM (Hylander et al., 2005; Vinyard et al., 2005). This technique generates a representative statistic (the mean) for all cycles in the species, resulting in a binary observation on the presence/absence of triplets. It does not take into consideration both the inter-cycle and intra-cycle variance in the triplet motor pattern. The second method estimates from the available data the proportion of cycles per species following the triplet motor pattern and whether the number of triplet cycles differs from that predicted at random. This method accounts for both inter-individual variation and intra-individual variation. It accounts for the fact that (1) multiple motor patterns produce the force and kinematics required to mechanically digest food during mastication; and (2) the motor pattern is continuously modified based on sensorimotor feedback, even within a single masticatory cycle. Therefore, the motor pattern changes not only within species but also within individuals, and even within individual cycles. The results of this study are summarized in Fig. 5.
Fig. 5.
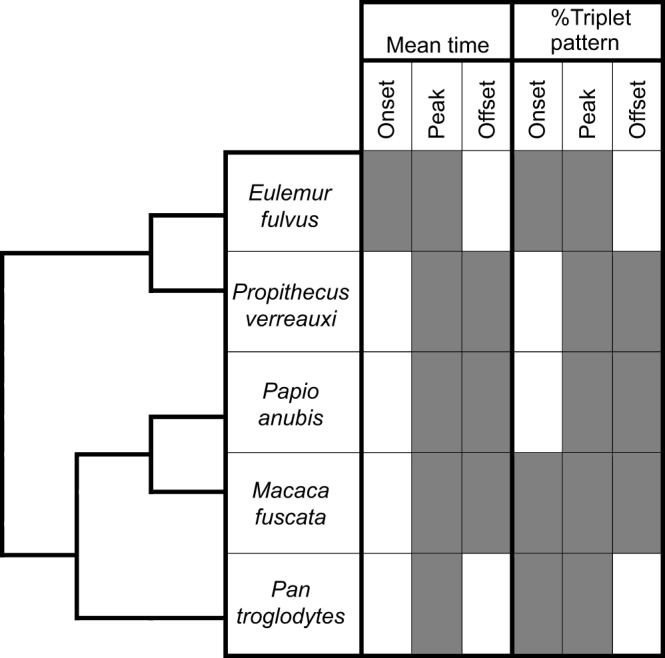
Testing the presence and prevalence of the triplet motor pattern in the five primate species. Data are the mean onset, peak and offset times and the percentage of cycles per species following the triplet motor pattern. Corroborated hypotheses are shown as shaded boxes.
The two methods evaluating whether the triplet motor pattern was present yielded similar results for E. fulvus, P. verreauxi and P. anubis. For M. fuscata, more cycles than expected by random probability follow the triplet motor pattern at onset. Out of a total of 834 macaque cycles analyzed, wSM (triplet II) reached onset before wPT (triplet I) in just 41 cycles. Based solely on the mean, M. fuscata do not follow the triplet motor pattern at onset, although 36% of all jaw elevator cycles comply with the triplet motor pattern at onset. Similarly, in P. troglodytes, significantly more cycles follow the triplet motor pattern at onset than expected by random probability. However, because 21% of cycles display this motor pattern, the trend is not captured by the mean. Hence, the mean onset, peak and offset times do not capture the variability in muscle onset, peak and offset times.
All five primate species studied exhibited the triplet motor pattern at peak activation in a significant proportion of cycles. These findings corroborate Weijs's (1994) triplet hypothesis at peak muscle activation only. This suggests that there may be a functional reason for maintenance of the triplet motor pattern at peak muscle activation in primates. Among the anthropoids, the two Old World monkeys analyzed, P. anubis and M. fuscata, also exhibited the triplet motor pattern at peak and offset. Moreover, presentation of the triplet motor pattern at peak and offset appears to be a convergent character shared by non-hominid anthropoids. Propithecus verreauxi, a strepsirrhine species that independently evolved a fused mandibular symphysis, also follows the triplet motor pattern at peak and offset but not at onset. Eulemur fulvus, the only species studied with an unfused symphysis, was the only species whose mean onset time followed the triplet motor pattern. Further studies including a larger range of species are needed to confirm these findings; however, we hypothesize that the presence of the triplet motor pattern at peak and offset is a convergent trait for non-hominid primates with a fused symphysis.
Pan troglodytes, the only hominid species studied, exhibits the triplet motor pattern only at mean peak time and is the only anthropoid species studied that does not have more triplet cycles at offset than expected by random probability. While chimps have never been previously tested for the triplet motor pattern, their close relative, Homo sapiens, does follow the triplet motor pattern at peak muscle activation (Møller, 1966; Langenbach and Hannam, 1999; Williams et al., 2011). Incidentally, P. troglodytes is both the only hominid species included in the present study and the only species with a fused mandibular symphysis that does not demonstrate the triplet motor pattern at offset.
These findings demonstrate that the standard practice of calculating the mean onset, peak and offset time does not capture the variation in muscle activation patterns used by primates at different times in the cycle and in different cycles. What is the significance of this variation? One possibility is that the triplet motor pattern acts as an ‘attractor’ to the masticatory system; i.e. a stable point or pattern of activity toward which a system progresses either spontaneously or in response to stimulus and/or feedback (Yuste et al., 2005). An attractor can be visualized as a basin in a landscape that represents the system's activity (Yuste et al., 2005). This hypothesis suggests that if all external forces and factors acting on the system were constant, the triplet motor pattern would be one among a discrete and finite set of motor patterns observed. However, modifications to the motor pattern may be necessary to maintain kinematics given changing conditions, such as food bolus, condition and position (Ross and Iriarte-Diaz, 2014). The above hypothesis is bolstered by the fact that in four out of the species, the permutations bSM, wPT, wSM, bPT and wPT, bSM, wSM, bPT occur more frequently than expected by random probability at at least two time points during the chewing cycle. These permutations were followed in frequency by wPT, wSM, bSM, bPT, which was observed more frequently than expected at onset, peak and offset in three out of five species, and the permutation bSM, wSM, wPT, bPT, which occurred more frequently than expected by random probability during at least two time points in three out of five species.
As a result, the triplet motor pattern may be one of multiple motor patterns that produce a given pattern of kinematics and force production, especially during offset and onset. Currently, it is unclear what conditions contribute to the triplet motor pattern or its alternatives being generated/selected by the central nervous system. These conditions may include variations in kinematics related to food type and temporal position in the masticatory sequence (early versus late) (Reed and Ross, 2010). Nonetheless, in all four of the most frequently observed motor patterns, wPT fires before bPT. PT is thought to have less of a wishboning effect than SM and MP and is thought to produce a predominantly vertical and posteriorly directed force (Hylander and Johnson, 1994). Hence, cycles in which wPT fires before bPT but the wSM fires before bPT, or cycles where both PT fire before both SM would be hypothesized to have decreased wishboning and transverse rotation and may be utilized when increased transverse forces are not needed.
Alternatively, there might not be obvious or predominant relationships between food type-related variance in jaw kinematics and muscle motor patterns because of redundancy in the masticatory system – multiple patterns of muscle activation can generate similar patterns of force production and kinematics (Van Eijden et al., 1990; Vinyard et al., 2008). This hypothesis is supported in species where the mean onset, peak or offset times do not follow the triplet motor pattern but the proportion of cycles that follow the triplet motor pattern is higher than expected by random probability. One or two jaw elevator muscles reaching onset, peak or offset milliseconds too early or too late modify the motor pattern but may not appreciably affect the kinematics. In this case, motor patterns could only be considered to be conserved if the variation in kinematics is disproportionately larger than the variation in motor patterns: to address this possibility, future work should examine variation in muscle activity patterns relative to variation in jaw kinematics. Previous workers have suggested, based on qualitative observations, that jaw kinematics do vary more than motor patterns (Hiiemae, 1978), but this has yet to be proven quantitatively. If the variation in kinematics is less than or proportional to the variation in motor pattern, then it is possible that the central nervous system is not wedded to specific motor patterns but is more concerned with modulating force production and kinematics to achieve specific goals. If the variation in jaw kinematics is equal to the variation in motor patterns, the goal itself may be changing during the masticatory sequence. Directed changes in force production and kinematics may be correlated with changing bolus properties and points of tooth contact.
It has been suggested that mammals display broad similarities in motor patterns during mastication (Hiiemae, 1978; Bramble and Wake, 1985; Weijs, 1994). Asymmetrical activity of the bilateral jaw elevator muscles (Herring and Scapino, 1973; Herring, 1976; Herring et al., 1979; Gorniak, 1977, 1985; Weijs and Dantuma, 1980) was reified into a triplet motor pattern, ancestral for mammals, and modified by natural selection (Weijs, 1994). Many workers in the field have used the mean peak activation time of triplet muscles to test for the presence of triplets in a variety of mammals. The current study found that the mean peak activity times for the jaw elevator muscles follow the triplet motor pattern in all five species studied. The prevalence of the triplet motor pattern is also greatest at peak muscle activation compared with onset and offset. However, there was variability in the prevalence of the triplet motor pattern between species (ranging from 20% in P. troglodytes to 81% in P. verreauxi at peak muscle activation). Flexibility in muscle activation patterns indicates that the jaw elevator motor pattern is not a fixed binomial trait. There is variability between cycles and the motor pattern can be modified between onset, peak and offset. Further studies are needed to understand the implications of this variability for motor control by the CNS.
Acknowledgements
Thanks to Jose Iriarte-Diaz for technical support and advice and to Christine Wall for answering questions regarding data available on the FEED database. Special thanks to Susan Larson and Jack T. Stern Jr for assistance collecting the Pan data included in this paper.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: Y.R., C.F.R.; Methodology: Y.R., C.F.R.; Software: Y.R.; Validation: C.F.R.; Formal analysis: Y.R.; Investigation: Y.R.; Resources: C.F.R.; Data curation: Y.R., C.F.R.; Writing - original draft: Y.R.; Writing - review & editing: Y.R., C.F.R.; Supervision: C.F.R.; Funding acquisition: C.F.R.
Funding
This research was supported by the National Institutes of Health (RO1DE023816) and the National Science Foundation (DGE-0903637). Deposited in PMC for release after 12 months.
References
- Binder M. D., Hirokawa N. and Windhorst U. (ed.). (2009). Encyclopedia of Neuroscience (Vol. 3166). Berlin, Heidelberg: Springer. [Google Scholar]
- Bramble D. M. and Wake D. B. (1985). Feeding mechanisms in lower tetrapods. In Functional Vertebrate Morphology (ed. Hildebrand M., Bramble D. M., Liem K. F. and Wake D. B.), pp. 230-261. Cambridge: Belknap Press. [Google Scholar]
- Crompton A. W., Barnet J., Lieberman D. E., Owerkowicz T., Skinner J. and Baudinette R. V. (2008a). Control of jaw movements in two species of macropodines (Macropus eugenii and Macropus rufus). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 150, 109-123. 10.1016/j.cbpa.2007.10.015 [DOI] [PubMed] [Google Scholar]
- Crompton A. W., Lieberman D. E., Owerkowicz T., Baudinette R. V. and Skinner J. (2008b). Motor control of masticatory movements in the Southern hairy-nosed wombat (Lasiorhinus latifrons). In Primate Craniofacial Function and Biology. Developments In Primatology: Progress and Prospects (ed. Vinyard C., Ravosa M. J. and Wall C.), pp. 83-111. Boston, MA: Springer; 10.1007/978-0-387-76585-3_5 [DOI] [Google Scholar]
- Crompton A. W., Owerkowicz T. and Skinner J. (2010). Masticatory motor pattern in the koala (Phascolarctos cinereus): a comparison of jaw movements in marsupial and placental herbivores. J. Exp. Zool. A Ecol. Genet. Physiol. 313A, 564-578. 10.1002/jez.628 [DOI] [PubMed] [Google Scholar]
- Davis J. S. (2014). Functional morphology of mastication in musteloid carnivorans. PhD thesis, Ohio University. [Google Scholar]
- Gorniak G. C. (1977). Feeding in golden hamsters, Mesocricetus auratus. J. Morphol. 154, 427-458. 10.1002/jmor.1051540305 [DOI] [PubMed] [Google Scholar]
- Gorniak G. C. (1985). Trends in the actions of mammalian masticatory muscles. Am. Zool. 25, 331-338. 10.1093/icb/25.2.331 [DOI] [Google Scholar]
- Herring S. W. (1976). The dynamics of mastication in pigs. Arch. Oral Biol. 21, 473-480. 10.1016/0003-9969(76)90105-9 [DOI] [PubMed] [Google Scholar]
- Herring S. W. and Scapino R. P. (1973). Physiology of feeding in miniature pigs. J. Morphol. 141, 427-460. 10.1002/jmor.1051410405 [DOI] [PubMed] [Google Scholar]
- Herring S. W., Grimm A. F. and Grimm B. R. (1979). Functional heterogeneity in a multipinnate muscle. Am. J. Anat. 154, 563-575. 10.1002/aja.1001540410 [DOI] [PubMed] [Google Scholar]
- Hiiemae K. M. (1976). Masticatory movements in primitive mammals. In Mastication (ed. Anderson D. J. and Matthews B.), pp. 105-118. Bristol: John Wright & Sons Ltd. [Google Scholar]
- Hiiemae K. M. (1978). Mammalian mastication: a review of the activity of jaw muscles and the movements they produce in chewing. In Development, Function and Evolution of Teeth (ed. Butler P. M. and Joysey K.), pp. 359-398. London: Academic Press. [Google Scholar]
- Hylander W. L. (1984). Stress and strain in the mandibular symphysis of primates: a test of competing hypotheses. Am. J. Phys. Anthropol. 64, 1-46. 10.1002/ajpa.1330640102 [DOI] [PubMed] [Google Scholar]
- Hylander W. L. and Johnson K. R. (1985). Temporalis and masseter muscle function during incision in macaques and humans. Int. J. Primatol. 6, 289-322. 10.1007/BF02745501 [DOI] [Google Scholar]
- Hylander W. L. and Johnson K. R. (1994). Jaw muscle function and wishboning of the mandible during mastication in macaques and baboons. Am. J. Phys. Anthropol. 94, 523-547. 10.1002/ajpa.1330940407 [DOI] [PubMed] [Google Scholar]
- Hylander W. L., Johnson K. R. and Crompton A. W. (1987). Loading patterns and jaw movements during mastication in Macaca fascicularis: a bone-strain, electromyographic, and cineradiographic analysis. Am. J. Phys. Anthropol. 72, 287-314. 10.1002/ajpa.1330720304 [DOI] [PubMed] [Google Scholar]
- Hylander W. L., Ravosa M. J., Ross C. F., Wall C. E. and Johnson K. R. (2000). Symphyseal fusion and jaw-adductor muscle force: an EMG study. Am. J. Phys. Anthropol. 112, 469-492. [DOI] [PubMed] [Google Scholar]
- Hylander W. L., Vinyard C. J., Ravosa M. J., Ross C. R., Wall C. E. and Johnson K. R. (2004). Jaw elevator force and symphyseal fusion. Development 11, 4. [Google Scholar]
- Hylander W. L., Wall C. E., Vinyard C. J., Ross C., Ravosa M. R., Williams S. H. and Johnson K. R. (2005). Temporalis function in anthropoids and strepsirrhines: an EMG study. Am. J. Phys. Anthropol. 128, 35-56. 10.1002/ajpa.20058 [DOI] [PubMed] [Google Scholar]
- Hylander W. L., Vinyard C. J., Wall C. E., Williams S. H. and Johnson K. R. (2011). Functional and evolutionary significance of the recruitment and firing patterns of the jaw adductors during chewing in verreaux's sifaka (Propithecus verreauxi). Am. J. Phys. Anthropol. 145, 531-547. 10.1002/ajpa.21529 [DOI] [PubMed] [Google Scholar]
- Langenbach G. E. J. and Hannam A. G. (1999). The role of passive muscle tensions in a three-dimensional dynamic model of the human jaw. Arch. Oral Biol. 44, 557-573. 10.1016/S0003-9969(99)00034-5 [DOI] [PubMed] [Google Scholar]
- Meredith R. W., Janečka J. E., Gatesy J., Ryder O. A., Fisher C. A., Teeling E. C., Goodbla A., Eizirik E., Simao T. L. L., Stadler T. et al. (2011). Impacts of the Cretaceous Terrestrial Revolution and KPg extinction on mammal diversification. Science 334, 521-524. 10.1126/science.1211028 [DOI] [PubMed] [Google Scholar]
- Møller E. (1966). The chewing apparatus. An electromyographic study of the action of the muscles of mastication and its correlation to facial morphology. Acta Physiol. Scand. 280, 1-229. [PubMed] [Google Scholar]
- Perelman P., Johnson W. E., Roos C., Seuánez H. N., Horvath J. E., Moreira M. A. M., Kessing B., Pontius J., Roelke M., Rumpler Y. et al. (2011). A molecular phylogeny of living primates. PLoS Genet. 7, e1001342 10.1371/journal.pgen.1001342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravosa M. J., Vinyard C. J., Gagnon M. and Islam S. A. (2000). Evolution of anthropoid jaw loading and kinematic patterns. Am. J. Phys. Anthropol. 112, 493-516. [DOI] [PubMed] [Google Scholar]
- Reed D. A. and Ross C. F. (2010). The influence of food material properties on jaw kinematics in the primate, Cebus. Arch. Oral Biol. 55, 946-962. 10.1016/j.archoralbio.2010.08.008 [DOI] [PubMed] [Google Scholar]
- Ross C. F. and Iriarte-Diaz J. (2014). What does feeding system morphology tell us about feeding? Ev. Anthropol. 23, 105-120. 10.1002/evan.21410 [DOI] [PubMed] [Google Scholar]
- Ross C. F., Baden A. L., Georgi J., Herrel A., Metzger K. A., Reed D. A., Schaerlaeken V. and Wolff M. S. (2010). Chewing variation in lepidosaurs and primates. J. Exp. Biol. 213, 572-584. 10.1242/jeb.036822 [DOI] [PubMed] [Google Scholar]
- Ross C. F., Iriarte-Diaz J. and Nunn C. L. (2012). Innovative approaches to the relationship between diet and mandibular morphology in primates. Int. J. Primatol. 33, 632-660. 10.1007/s10764-012-9599-y [DOI] [Google Scholar]
- Van Eijden T. M. G. J., Brugman P., Weijs W. A. and Oosting J. (1990). Coactivation of jaw muscles: recruitment order and level as a function of bite force direction and magnitude . J. Biomech. 23, 475-485. 10.1016/0021-9290(90)90303-K [DOI] [PubMed] [Google Scholar]
- Vinyard C. J., Williams S. H., Wall C. E., Johnson K. R. and Hylander W. L. (2005). Jaw-muscle electromyography during chewing in Belanger's treeshrews (Tupaia belangeri). Am. J. Phys. Anthropol. 127, 26-45. 10.1002/ajpa.20176 [DOI] [PubMed] [Google Scholar]
- Vinyard C. J., Wall C. E., Williams S. H., Johnson K. R. and Hylander W. L. (2006). Masseter electromyography during chewing in ring-tailed lemurs (Lemur catta). Am. J. Phys. Anthropol. 130, 85-95. 10.1002/ajpa.20307 [DOI] [PubMed] [Google Scholar]
- Vinyard C. J., Wall C. E., Williams S. H. and Hylander W. L. (2008). Patterns of variation across primates in jaw-muscle electromyography during mastication. Int. Comp. Biol. 48, 294-311. 10.1093/icb/icn071 [DOI] [PubMed] [Google Scholar]
- Wall C. E., Vinyard C. J., Williams S. H., Gapeyev V., Liu X., Lapp H. and German R. Z. (2011). Overview of FEED, the feeding experiments end-user database. Int. Comp. Biol. 51, 215-223. 10.1093/icb/icr047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weijs W. A. (1994). Evolutionary approach to masticatory motor patterns in mammals. In Advances in Comparative and Environmental Physiology, vol. 18 (ed. V. L. Bels, M. Chardon and P. Vandewalle), pp. 281-320. Berlin, Heidelberg: Springer-Verlag. [Google Scholar]
- Weijs W. A. and Dantuma R. (1980). Functional anatomy of the masticatory apparatus in the rabbit (Oryctolagus cuniculus L.). Netherlands J. Zool. 31, 99-147. 10.1163/002829680X00212 [DOI] [Google Scholar]
- Williams S. H., Vinyard C. J., Wall C. E. and Hylander W. L. (2007). Masticatory motor patterns in ungulates: a quantitative assessment of jaw-muscle coordination in goats, alpacas and horses. J. Exp. Zool. A Ecol. Genet. Physiol. 307A, 226-240. 10.1002/jez.362 [DOI] [PubMed] [Google Scholar]
- Williams S. H., Vinyard C. J., Wall C. E., Doherty A. H., Crompton A. W. and Hylander W. L. (2011). A preliminary analysis of correlated evolution in mammalian chewing motor patterns. Int. Comp. Biol. 51, 247-259. 10.1093/icb/icr068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R., MacLean J. N., Smith J. and Lansner A. (2005). The cortex as a central pattern generator. Nat. Rev. Neurosci. 6, 477 10.1038/nrn1686 [DOI] [PubMed] [Google Scholar]