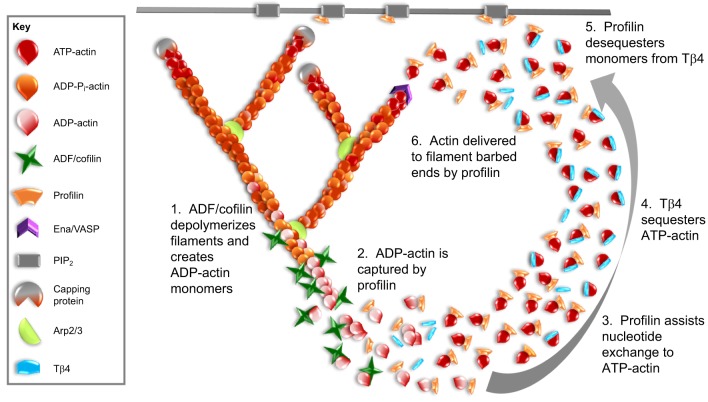
Fig. 2.
Profilin and Tβ4 regulate the monomer pool. This figure presents a model for how profilin and Tβ4 may work together to convert newly released actin monomers into a polymerization-competent pool that is then directed to re-polymerize into new filaments. After depolymerization of filaments by disassembly factors such as ADF/cofilin (1), profilin binds to the newly released monomers (2) due to its higher affinity for ADP-actin. Profilin induces nucleotide exchange (3), and the majority of monomers are transferred to Tβ4 (4), which has a 50-fold higher affinity for ATP-actin over ADP-actin and is present in the cell at higher concentrations than profilin. Tβ4 holds the monomers in a polymerization-competent pool. Owing to high rates of exchange, profilin de-sequesters monomers from Tβ4 (5) and delivers G-actin to barbed-end polymerases such as Ena/VASP (6). Profilin can also localize to the plasma membrane through an interaction with phosphatidylinositol 4,5-bisphosphate (PIP2). A profilin–Tβ4–G-actin ternary complex may assist in the transfer of G-actin from Tβ4 to profilin by providing an intermediate state, where the actin monomer does not dissociate from either protein, thereby preventing its spontaneous assembly into filaments.