Short abstract
Background
Fatigue is a major symptom of multiple sclerosis (MS) in patients, and it has been shown to improve with physical exercise. Although fingolimod might lessen fatigue, it is unclear how patients treated with fingolimod react to physical activity regarding fatigue.
Objective
This study evaluated the effect of an exercise intervention on fatigue in relapsing–remitting MS patients receiving fingolimod.
Methods
People with MS (PwMS) were randomized to either a structured internet-based exercise program (e-training) or no e-training intervention. The primary endpoint was the change in the Modified Fatigue Impact Scale (mFIS) after six months.
Results
The primary analysis showed no statistically significant difference between groups in the mFIS change. Subgroup analyses revealed a beneficial effect of physical exercise for PwMS with low aerobic capacity and with low aerobic capacity plus more severe fatigue. The incidence of adverse events was similar in both groups. No cardiovascular events were reported. The majority of PwMS were relapse free.
Conclusion
Physical exercise benefits on fatigue may depend on the physical capacity of the patient and requires individualized training. Consistent with previous studies, these results suggest that physical exercise generally does not impose a risk and that this holds true also for patients receiving fingolimod.
Trial registration: ClinicalTrials.gov, NCT01490840.
Keywords: Multiple sclerosis, fatigue, physical exercise, training, fingolimod, aerobic capacity
Introduction
Fatigue is a common disabling symptom of multiple sclerosis (MS), which more than two-thirds of all people with MS (PwMS) judge to be one of the worst.1–3 It has been defined as “a subjective lack of physical and/or mental energy that is perceived by the individual or caregiver to interfere with usual and desired activities.”4 Fatigue has a huge impact on the quality of life (QoL) of PwMS.5 Therefore, interventions that reduce fatigue are of particular interest for this patient population.
Physical exercise is a promising intervention strategy to improve this multidimensional and complex phenomenon. Various mechanisms have been proposed to contribute to improving fatigue, including increased physical capacity and motor functioning, cardiovascular changes, neurotrophic or neuroendocrine changes, and improved depression.6 Several studies have demonstrated that PwMS benefit from physical exercise through improved physical fitness,7,8 walking ability,9 QoL10 and depression.11,12 Physical exercise is safe, overall well tolerated13,14 and not associated with higher disease activity.15 Regular physical activity may even have positive long-term effects on disability progression.16
A recent Cochrane review17 and another meta-analysis18 based on randomized, controlled trials revealed significant improvements in fatigue among PwMS following physical exercise interventions. The overall effect sizes were small to moderate. Endurance training, a combination of endurance and resistance training and other forms of exercise (e.g. yoga, tai chi) were particularly effective in reducing fatigue.17,18 However, positive effects on fatigue were not consistently found across studies, and there was considerable variation in the magnitude of effects. It was assumed that this variation was possibly due to the variability in the exercise programs with respect to intensity, frequency, and duration as well as the lack of a preselection of study populations by presence of fatigue in most of the studies.18,19
Further major limitations of previous studies are that fatigue was rarely defined as the primary study goal, sample sizes were small and intervention periods short (mainly four to 12 weeks).17–19 One reason might be the location-dependent nature of interventions at a clinic or training center.20 Various examples show that internet-based interventions contribute to positive changes in physical activity.21–23 The herein applied internet-based intervention has been shown to be a feasible approach24 to deliver exercise therapy to a large population over a wide area (n = 126), and facilitate individually tailored exercise support with significant improvements in physical fitness and activity among PwMS.25 Other internet-based physical activity interventions support this finding in PwMS.26,27
A recent review on safety of exercise in MS concluded that exercise training was not associated with an increased risk of relapse, and also the risk of adverse events (AEs) was not higher than in healthy populations.13 Thus, an internet-delivered home-based exercise intervention can be considered safe, especially if specific safety instructions, supervision by trained and experienced therapists and prior physical examinations are ensured.25,28
In addition to interventional strategies, optimal fatigue management has to consider a range of potential influencers. Next to psychological distress and depressive symptoms that have been shown to correlate with fatigue, the medication used by PwMS might also induce or enhance symptoms of fatigue.29 This includes analgesics, antihistamines, and muscle relaxants, as well as disease-modifying therapies themselves.29 For example, beta-interferons are possibly associated with fatigue, while a switch to other medications, such as glatiramer acetate or fingolimod, has been shown to improve symptoms.30,31
Fingolimod (FTY720, Gilenya®) is an oral, once-daily immunomodulatory drug approved for the treatment of relapsing–remitting MS (RRMS) in the United States, Europe, and other regions. Fingolimod’s mode of action is characterized by sequestration of lymphocytes in the lymph nodes. Fatigue is not known to be a side effect of fingolimod; the drug rather improves symptoms of fatigue. Despite the generally well-understood efficacy and safety profile of fingolimod, it is unclear how patients on fingolimod react to increased levels of physical activity in a structured exercise program, in particular with regard to fatigue and safety.
The objective of the present study was to evaluate the effect of exercise intervention on MS-related fatigue in people with RRMS receiving fingolimod. The focus was on endurance and strength exercise. In addition, satisfaction and acceptability of this intervention approach is assessed. Further, the study delivers valuable information on the safety of fingolimod in physically active patients.
Methods
Study design and procedures
The present study was a prospective, multicenter, randomized, controlled parallel-group study on the effect of physical activity in fingolimod-treated patients with RRMS (PACE study). Patients were recruited at MS outpatient centers in Germany.
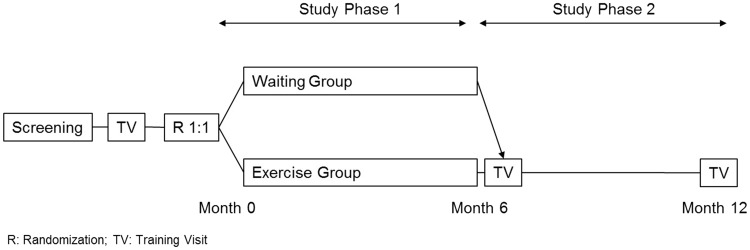
In study phase 1, participants were randomized 1:1 to receive either a structured e-training intervention (e-training group) or no physical intervention (waiting group). After six months, the primary endpoint of the study was assessed. In study phase 2, participants in the e-training group had the option to continue for an additional six months, while participants in the waiting group could opt to start the e-training program (Figure 1). Medical data were collected at the study sites at baseline, after 3, 6, 9, and 12 months, and AEs were additionally collected by periodical phone contact. Physical capacity and fitness parameters were assessed at baseline, and after six and 12 months at a central training center (training visit).
Figure 1.
Study design.
The study was conducted according to the principles of the Declaration of Helsinki and the study protocol was approved by the independent ethics committee or institutional review board at each of the participating study sites. The study was registered at ClinicalTrials.gov (NCT01490840). From each patient, written informed consent was obtained before conducting any study-specific procedures.
Participants
Eligible participants were aged 18 to 65 years with an established diagnosis of RRMS. To avoid confounding effects of background disease-modifying therapy on the outcomes, only patients who received stable fingolimod therapy for at least one month prior to screening were included. A maximum Expanded Disability Status Scale (EDSS) score of 3.5 was allowed, and the mFIS score had to be above 14 at screening. Patients had to be neurologically stable with no evidence of relapse within 30 days prior to recruitment. Patients were required to have access to the internet in order to enter the e-training platform.
Key exclusion criterion was prior treatment with immunosuppressive or immunomodulating medications within one to three months before randomization, depending on the medication (except for cladribine, which was not allowed at any time before randomization) to avoid medication-induced bias. Further, patients with a cardiovascular risk profile, severe respiratory or pulmonary disease, or any clinically relevant internal disease or orthopedic diseases that could interfere with exercise were excluded to ensure that patients were able to safely and effectively follow a training program.
Intervention
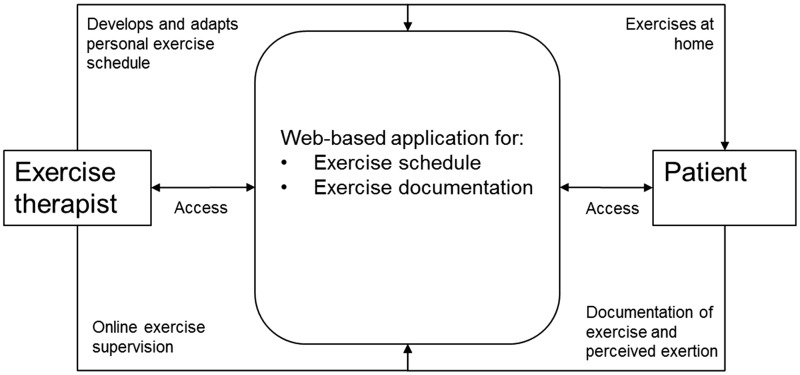
The e-training intervention employed a web-based application to administer an adaptive and individualized exercise protocol (Figure 2). The exercise intervention was home based and supervised via the internet by a physiotherapist or exercise therapist with experience in the prevention and rehabilitation setting with different indications including MS. The therapists were additionally trained with regard to all study and prescription processes. Target exercise intensity was moderate and progression was regulated by each participant’s subjective, perceived exertion, which was rated between 6 and 20 on the Borg Scale.32 A more detailed intervention description is available here25 and as supplementary material (Supplement 1).
Figure 2.
Interaction between patient and exercise therapist via web-based training application.
The individual exercise schedules comprised strengthening exercises twice a week and endurance training once a week. Balance or core stability exercise could be added. The personal exercise schedule and the comprised exercises were explained in a two-day on-site introductory group session at the beginning of the intervention period. Participants documented each exercise session via a web-based application (duration, type of exercises, number of repetitions, and sets, perceived exertion) and used an electronic exercise diary that could be supervised by the exercise therapist.
Outcomes and assessments
The primary endpoint was the effect of physical exercise on fatigue measured as change from baseline to month 6 using the Modified Fatigue Impact Scale (mFIS).33 Secondary outcomes included the effect of structured physical e-training on fatigue using the Würzburg Fatigue Inventory for MS scale (WEIMuS), depressive symptoms using the Beck Depression Inventory (BDI-II), QoL by means of the Hamburg Quality of Life Questionnaire in MS (HAQUAMS), functional capacity of lower extremities (sit to stand), maximum isokinetic, dynamic leg strength (Isomed 2000, D&R Ferstl GmbH, Germany), maximum isometric trunk strength (M3 Diagnos, Schnell GmbH, Germany) and aerobic capacity measured by a graded exercise test on a treadmill using spiroergometry (Cardinal Health, Germany). To reduce risk of bias, assessments were standardized, and assessors were blinded and trained according to the assessment procedures.
MS disease course was assessed by EDSS34 and Multiple Sclerosis Functional Composite (MSFC)35,36 scores. Additionally, compliance with e-training was calculated as the percentage of exercise sessions documented by the patient in relation to the number of planned exercise sessions.
Feasibility and acceptability was assessed via a short online questionnaire at the end of the study. It covered the aspects of meaningfulness and acceptance of an internet-based approach, satisfaction with the usability and design of the internet platform, quality of therapeutic support and demand to perform the strength and endurance sessions as well as interest in continuing the intervention after the end of the study. Participants were asked to answer the online questionnaire at the central training center at the last assessment visit to ensure a high retention rate. Thus, participants were asked after six or 12 months of training support.
Safety was assessed as the incidence of (serious) AEs by the Medical Dictionary for Regulatory Activities system organ class and preferred term. AEs were defined as the appearance or worsening of any undesirable sign, symptom, or medical condition occurring after study drug intake even if the event was not considered to be related to drug therapy.
Sample size and randomization
A sample size of 90 in each group would have had 80% power to detect a difference in means of 3.8 points, assuming that the common standard deviation (SD) is 9.05, using a two group t-test with a 0.05 two-sided significance level. Assuming a dropout rate of 20%, 226 patients (113 per arm) were planned to be randomized to this study, which required screening approximately 272 patients considering a screening failure rate of 20%.
Randomization was stratified by aerobic capacity. The allocation sequence was created centrally using SAS 9.2 statistical software. The allocation sequence was concealed from the investigator enrolling and assessing participants in sequentially numbered, sealed, opaque envelopes. Allocation of participants was performed by the investigator at the study centers.
Statistical methods
The primary endpoint tested the superiority of the e-training intervention based on an analysis of covariance (ANCOVA) model with the factors intervention group and gender. The model was adjusted for the covariates baseline mFIS, baseline EDSS, and baseline aerobic capacity. Missing values were replaced by the last observation carried forward method. The primary endpoint analysis was based on the modified full analysis set (modified FAS). The significance level of the confirmatory analysis was pre-specified at α = 0.05 (two sided). A sensitivity analysis considered the FAS. The FAS consisted of all patients who had at least one post-baseline assessment for mFIS and followed the intention-to-treat principle. The modified FAS consisted of all patients from the FAS for whom sufficient exercise compliance was established, i.e. who had completed 70% of the scheduled exercise sessions during month 1 to 6, or more than 80% during month 5 to 6.
Analyses of the secondary endpoints were also using ANCOVA models. In addition to pre-specified analyses, exploratory post-hoc analyses were performed on subgroups of baseline maximal aerobic capacity (VO2max) and baseline mFIS. The thresholds were defined as the one-third and two-thirds quantiles for subgroups defined by VO2max and the median for the subgroups defined by both VO2max and mFIS (to account for fatigue as a cofactor of physical fitness). The safety analysis set consisted of all enrolled patients for whom safety information was collected. All analyses have been performed using SAS 9.2 statistical software.
Results
Study population
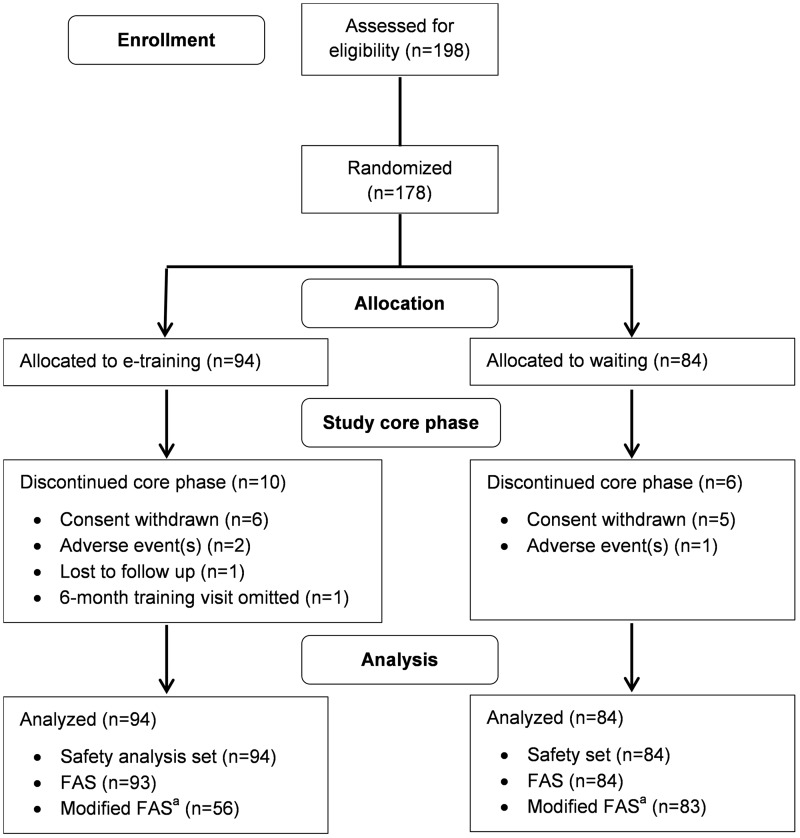
A total of 198 PwMS were screened for study eligibility at 32 German study centers. In total, 20 patients were screening failures, thus 178 patients were randomized to the e-training group (N = 94) or the waiting group (N = 84). Between 2011 and 2014, 162 PwMS completed phase I of the study. Reasons for premature discontinuations were withdrawal of consent, AEs, loss to follow-up, and omission of the six-month training center visit (Figure 3). The study was terminated early because of recruitment saturation. All randomized PwMS were included in the safety analysis set. A total of 177 PwMS were included in the FAS. The modified FAS consisted of 139 PwMS with sufficient exercise compliance (Figure 3). Both groups were comparable regarding demographics and baseline disease characteristics (Tables 1 and 2).
Figure 3.
Flowchart of patient disposition and analysis populations.aThe modified full analysis set (FAS) (used for primary analysis) consisted of all patients from the FAS for whom sufficient training compliance was established (see main text for details).
Table 1.
Demographics and baseline characteristics (FAS).
| E-training (N = 93) | Waiting (N = 84) | Total (N = 177) | |
|---|---|---|---|
| Mean ± SD/n (%) | Mean ± SD/n (%) | Mean ± SD/n (%) | |
| Age (years) | 40.9 ± 10.4 | 39.4 ± 8.7 | 40.2 ± 9.6 |
| Gender | |||
| Male | 29 (31.2) | 27 (32.1) | 56 (31.6) |
| Female | 64 (68.8) | 57 (67.9) | 121 (68.4) |
| Time since first MS diagnosis (years) | 8.0 ± 7.1 | 9.2 ± 7.2 | 8.6 ± 7.1 |
| Time since first MS symptoms (years) | 10.4 ± 8.9 | 11.4 ± 7.4 | 10.9 ± 8.2 |
| Number of relapses in the past six months | |||
| 0 | 93 (100.0) | 80 (95.2) | 173 (97.7) |
| 1 | 0 (0.0) | 4 (4.8) | 4 (2.3) |
| Baseline EDSS | 2.2 ± 1.0 | 2.2 ± 1.1 | 2.2 ± 1.0 |
| Baseline mFIS | 30.6 ± 14.9 | 34.4 ± 13.8 | 32.4 ± 14.5 |
| Baseline aerobic capacityVO2max (ml/min/kg) | 29.8 ± 6.4 | 29.7 ± 6.0 | 29.8 ± 6.2 |
EDSS: Expanded Disability Status Scale; FAS: full analysis set; mFIS: Modified Fatigue Impact Scale; MS: multiple sclerosis; SD: standard deviation.
Table 2.
Demographics and baseline characteristics (modified FAS).
| E-training (N = 56) | Waiting (N = 83) | Total (N = 139) | |
|---|---|---|---|
| Mean ± SD/n (%) | Mean ± SD/n (%) | Mean ± SD/n (%) | |
| Age (years) | 42.2 ± 10.2 | 39.3 ± 8.7 | 40.5 ± 9.4 |
| Gender | |||
| Male | 13 (23.2) | 26 (31.3) | 39 (28.1) |
| Female | 43 (76.8) | 57 (68.7) | 100 (71.9) |
| Time since first MS diagnosis (years) | 7.7 ± 6.1 | 9.3 ± 7.2 | 8.6 ± 6.8 |
| Time since first MS symptoms (years) | 10.7 ± 9.1 | 11.5 ± 7.4 | 11.2 ± 8.1 |
| Number of relapses in the past six months | |||
| 0 | 56 (100.0) | 79 (95.2) | 135 (97.1) |
| 1 | 0 (0.0) | 4 (4.8) | 4 (2.9) |
| Baseline EDSS | 2.2 ± 1.0 | 2.2 ± 1.1 | 2.2 ± 1.0 |
| Baseline mFIS | 30.5 ± 14.6 | 34.7 ± 13.7 | 33.0 ± 14.1 |
| Baseline aerobic capacityVO2max (ml/min/kg) | 29.9 ± 5.9 | 29.7 ± 6.0 | 29.8 ± 6.0 |
EDSS: Expanded Disability Status Scale; FAS: full analysis set; mFIS: Modified Fatigue Impact Scale; MS: multiple sclerosis; SD: standard deviation.
Compliance with e-training
Compliance with e-training (i.e. percentage of documented vs. planned exercise sessions) was highly variable among PwMS and ranged from 0 to 442.0%. This means that in addition to some patients with a very poor or even zero compliance, some patients had overachieved. Mean compliance was 82.4 ± 64.1% in the FAS. Based on the online documentation, 39.8% of the patients in the FAS were noncompliant as per definition. Only 60.2% of PwMS were classified as compliant and included in the modified FAS. Mean compliance in the modified FAS was 111.5 ± 63.9%. On the other hand, 32.2% of patients in the modified FAS had documented ≤60 of 72 planned exercise sessions. Overall, the number of documented exercise sessions per month increased until month 3 but stagnated thereafter.
Effect of e-training on fatigue
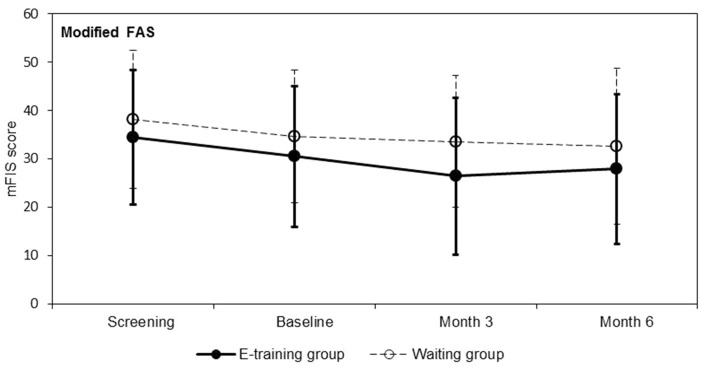
There was no statistically significant difference in the mean change of mFIS score from baseline to month 6 between the e-training and waiting group (Table 3). In both groups, the mFIS score changed only marginally during the course of the study (Figure 4).
Table 3.
Change in fatigue.
| N | BaselineMean ± SDa | Differencemonth 6—baselineMean (95% CI)b | p valueb | |
|---|---|---|---|---|
| mFIS | ||||
| Modified FAS | ||||
| E-training | 56 | 30.5 ± 14.6 | –3.57 (–6.81; –0.34) | |
| Waiting | 83 | 34.7 ± 13.7 | –2.10 (–4.69; 0.49) | |
| Difference e-training—waiting | –1.47 (–5.39; 2.44) | 0.4579 | ||
| FAS | ||||
| E-training | 93 | 30.6 ± 14.9 | –4.20 (–6.58; –1.83) | |
| Waiting | 84 | 34.4 ± 13.8 | –1.81 (–4.29; 0.67) | |
| Difference e-training—waiting | –2.40 (–5.71; 0.92) | 0.1554 | ||
| WEIMuS | ||||
| Modified FAS | ||||
| E-training | 56 | 28.4 ± 14.8 | –1.90 (–4.91; 1.11) | |
| Waiting | 83 | 30.4 ± 13.6 | –1.12 (–3.55; 1.31) | |
| Difference e-training—waiting | –0.78 (–4.42; 2.86) | 0.6723 | ||
| FAS | ||||
| E-training | 93 | 28.1 ± 14.9 | –2.94 (–5.19; –0.68) | |
| Waiting | 84 | 30.0 ± 13.9 | –0.89 (–3.24; 1.46) | |
| Difference e-training—waiting | –2.05 (–5.18; 1.09) | 0.1988 |
aUnadjusted raw means.
bResults from analysis of covariance model for difference in mFIS between baseline and month 6 with predictors baseline mFIS (for analysis of mFIS only), baseline WEIMuS (for analysis of WEIMuS only), baseline EDSS, baseline VO2max, sex and intervention group.
CI: confidence interval; EDSS: Expanded Disability Status Scale; FAS: full analysis set; mFIS: Modified Fatigue Impact Scale; N: number of patients; SD: standard deviation; WEIMuS: Würzburg Fatigue Inventory for MS scale.
The mFIS score ranges from 0 (not tired) to 84 (tired). The WEIMuS score ranges from 0 (not tired) to 68 (tired).
Figure 4.
Modified Fatigue Impact Scale (mFIS) score for fatigue (mean ± standard deviation) for modified full analysis set (FAS) (primary analysis). The mFIS score ranges from 0 (not tired) to 84 (tired).
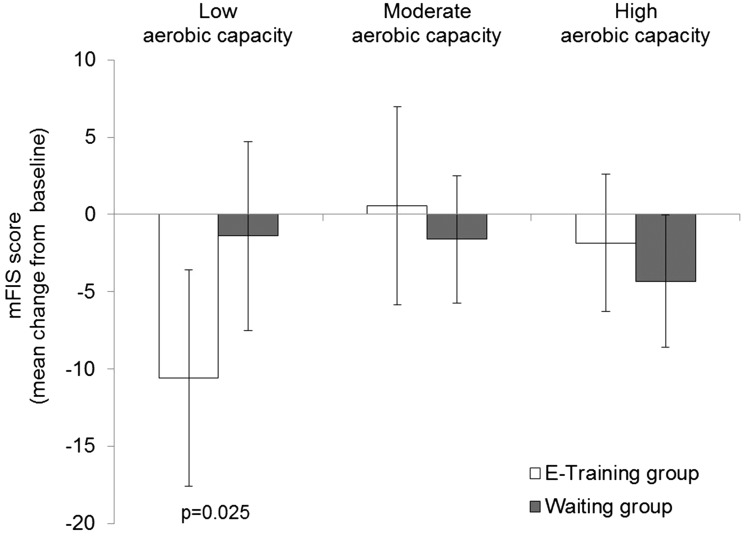
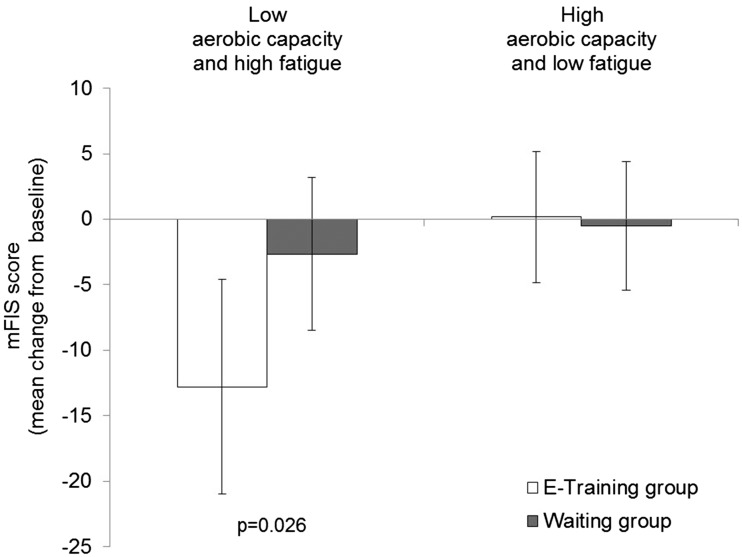
A significant difference between groups was observed in the subgroup of patients with low aerobic capacity (VO2max < 27 l/min/kg) at baseline (Figure 5 and Table 4). In this subgroup, the mean mFIS decreased in the e-training group while it remained nearly unchanged in the waiting group (p = 0.025). This between group difference was not observed in the subgroups with moderate (VO2max 27 to 31 l/min/kg) or high (VO2max > 31 l/min/kg) aerobic capacity (Figure 5 and Table 4). Furthermore, a significant effect (p = 0.026) of the intervention was also found in the subgroup of low aerobic capacity (VO2max ≤ 30 l/min/kg) plus high fatigue (mFIS>32), but not in the subgroup of high aerobic capacity (VO2max > 31 l/min/kg) plus low fatigue (Figure 6 and Table 4).
Figure 5.
Mean difference from baseline in Modified Fatigue Impact Scale (mFIS) score for fatigue (mean ± 95% confidence interval) for modified full analysis set (FAS) by subgroup of baseline aerobic capacity. Low aerobic capacity: VO2max < 27 l/min/kg; moderate aerobic capacity: VO2max 27 to 31 l/min/kg; high aerobic capacity: VO2max > 31 l/min/kg.
Table 4.
Change in fatigue (mFIS score) in subgroups (modified FAS).
| Fatigue (mFIS score) |
||||
|---|---|---|---|---|
| SubgroupIntervention group | N | BaselineMean ± SDa | Differencemonth 6—baselineMean (95% CI)b | p valueb |
| Low aerobic capacity (VO2max < 27 l/min/kg) | ||||
| E-training | 16 | 36.0 ± 15.4 | –10.60 (–17.60; –3.54) | |
| Waiting | 26 | 37.3 ± 12.5 | –1.40 (–7.53; 4.72) | |
| Difference e-training—waiting | –9.19 (–17.20; –1.22) | 0.025 | ||
| Moderate aerobic capacity(VO2max 27 to 31 l/min/kg) | ||||
| E-training | 17 | 28.1 ± 13.6 | 0.58 (–5.84; 7.00) | |
| Waiting | 33 | 34.0 ± 13.6 | –1.61 (–5.72; 2.50) | |
| Difference e-training—waiting | 2.19 (–4.88; 9.26) | 0.536 | ||
| High aerobic capacity (VO2max > 31 l/min/kg) | ||||
| E-training | 23 | 28.4 ± 14.2 | –1.84 (–6.28; 2.60) | |
| Waiting | 24 | 32.8 ± 15.2 | –4.33 (–8.61; –0.05) | |
| Difference e-training—waiting | 2.49 (–3.70; 8.68) | 0.422 | ||
| Low aerobic capacity (VO2max ≤ 30 l/min/kg)*high fatigue (mFIS > 32) | ||||
| E-training | 13 | 46.0 ± 9.1 | –12.80 (–21.00; –4.56) | |
| Waiting | 30 | 44.9 ± 8.2 | –2.65 (–8.48; 3.18) | |
| Difference e-training—waiting | –10.10 (–19.00; –1.27) | 0.026 | ||
| High aerobic capacity (VO2max > 30 l/min/kg)*low fatigue (mFIS ≤ 32) | ||||
| E-training | 16 | 20.0 ± 5.9 | 0.17 (–4.84; 5.19) | |
| Waiting | 16 | 22.1 ± 8.1 | –0.52 (–5.42; 4.38) | |
| Difference e-training—waiting | 0.69 (–6.54; 7.93) | 0.846 | ||
aUnadjusted raw means.
bResult from analysis of covariance model for difference in mFIS between baseline and month 6 with predictors baseline EDSS, mFIS (only for subgroups defined by aerobic capacity alone), sex and intervention group.
CI: confidence interval; EDSS: Expanded Disability Status Scale; FAS: full analysis set; mFIS: Modified Fatigue Impact Scale; N: number of patients; SD: standard deviation; WEIMuS: Würzburg Fatigue Inventory for MS scale.
The mFIS score ranges from 0 (not tired) to 84 (tired).
Figure 6.
Mean difference from baseline in Modified Fatigue Impact Scale (mFIS) score for fatigue (mean ± 95% confidence interval) for modified full analysis set (FAS) by subgroups of baseline aerobic capacity and fatigue. Low aerobic capacity (VO2max ≤ 30 l/min/kg) and high fatigue (mFIS > 32); high aerobic capacity (VO2max > 30 l/min/kg) and low fatigue (mFIS ≤ 32).
Effect on secondary outcomes
Fatigue as assessed by the WEIMuS showed a numerical improvement for both treatment groups (Table 3). The BDI-II improved by –2.62 points (95% confidence interval (CI) (–4.42; –0.81)) in the e-training group and –1.97 points (95% CI (–3.43; –0.52)) in the waiting group. The ANCOVA analysis did not show a statistically significant difference between both groups for both endpoints.
Health-related QoL assessed by the HAQUAMS total score did not change remarkably in the treatment groups and no difference between both was detected. This was also found for the subscale scores “fatigue/thinking” and “mobility lower limb.” Descriptively, a slight difference was observed regarding the change in the subscale score “mobility upper limb” (p = 0.0432).
For all measurements of muscular strength as well as for change in aerobic capacity as measured by spiroergometry, no relevant differences between both interventions were detected. The course of the exploratory endpoints EDSS and MSFC indicated no notable changes.
Feasibility
Overall, 129 (68% women, 32% men) people of 178 randomized participants answered the questionnaire. Of these, 61 persons (47%) were assessed after six months and 68 (53%) individuals after 12 months of exercise training. The mean age of the participants was 41.4 years (SD 10.0). Results on satisfaction and acceptability of the intervention are presented in Table 5.
Table 5.
Results of the feasibility and acceptance assessment.
| N | Mean ± SD1 | |
|---|---|---|
| Usability in general2 | 129 | 2.34 ± (.94 ) |
| Usability - graphical appeal3 | 126 | 4.12 ± (.98) |
| Usability - problems with the software4 | 127 | 2.31 ± (.93) |
| Therapeutic support - satisfaction with the therapist and their support at the introductory group session2 | 128 | 1.4 ± (.64) |
| Therapeutic support - satisfaction with the training support2 | 128 | 1.4 ± (.66) |
| Therapeutic support - satisfaction with the support at the central assessment center2 | 128 | 1.4 ± (.56) |
| Satisfaction about the quality of the information about the internet-based training and to independently conduct the training at home at the introductory group session3 | 128 | 4.4 ± (.72 ) |
| Usefulness and meaningfulness of an internet-supported training3 | 126 | 4.4 ± (.89) |
| Interest in the continuation of the training3 | 127 | 3.9 ± (1.1) |
N: number of respondents.
1SD: Standard Deviation
21 - very good to 5 - very bad.
31 - not at all to 5 - yes, very much
41 - never to 5 - always
AEs
The overall incidence of AEs over the six-month core study phase, irrespective of a causal relationship to drug therapy, was 58.5% in the exercise group and 60.7% in the waiting group. AEs of special interest are displayed in Table 6. No cardiovascular events were reported (Table 6). In both intervention groups, the majority of PwMS were relapse free (e-training group: 89.4%, waiting group: 95.2%).
Table 6.
Incidence of adverse events (all events and events of special interest) (safety analysis set).
| E-training (N = 94) | Waiting (N = 84) | |
|---|---|---|
| n (%) | n (%) | |
| Any adverse event | 55 (58.5) | 51 (60.7) |
| Cardiac disorders | – | – |
| Sleep disorder | 9 (9.6) | – |
| Respiratory, thoracic and mediastinal disorders | 1 (1.1) | 5 (6.0) |
| Vascular disorders | 1 (1.1) | 5 (6.0) |
| Any serious adverse event | – | 5 (6.0) |
| Cardiac disorders | – | – |
| Gamma-glutamyl transferase increased | – | 1 (1.2) |
Discussion
The present study did not detect a significant effect of a six-month structured e-training program on fatigue in patients with RRMS in general. However, the exercise program reduced fatigue in subgroups of PwMS with low aerobic capacity at baseline and PwMS with low aerobic capacity plus high fatigue at baseline, as shown by a post-hoc analysis.
Despite the large sample size in comparison with former exercise intervention studies, the primary analysis was underpowered, and thus results needs to be interpreted with caution.
Meta-analyses detected effects of exercise on MS-related fatigue, although there was considerable variation in content of exercise intervention modalities and effect magnitude.17,18 Endurance training, strength training or mixed interventions were particularly effective to reduce fatigue, partly contrasting with the results of the current study. Various potential pathophysiological pathways involved in fatigue and the positive effect of exercise on these have been proposed.6 However, the mechanisms remain poorly understood. Although the current study found no overall effect of exercise on MS-related fatigue, the findings of the subgroup analysis support the involvement of motor function and aerobic capacity pathways. This assumption was supported by the fact that the exercise program improved fatigue in PwMS with weaker physical capacity at baseline. PwMS with a low aerobic capacity and PwMS with low aerobic capacity plus more intense fatigue experienced an improvement in mFIS with a higher effect size compared to previous studies.19 Although it has to be kept in mind that these subgroups were defined only post hoc, the large difference between subgroups, the magnitude of the improvement in fatigue within certain subgroups, and the plausibility of the results suggest that the findings are valuable.
The lacking overall training effect might be explained by a subliminal training stimulus for participants who were physically fit at baseline with a mean EDSS of 2.2. Comparable studies on the effect of physical exercise on fatigue in PwMS also included individuals with greater impairment (e.g. EDSS up to 6) while the mean mFIS score was usually similar in earlier studies.14,19 The individual exercise schedules applied in this study were designed to create moderate exercise intensity for PwMS, but might have been too low in intensity to cause a measurable effect in a population of fairly fit and only weakly disabled PwMS.
An implication for the clinical context of these results is that, in addition to the degree of disability, the assessment of fatigue and aerobic capacity is valuable information in order to adapt the exercise training interventions to specific subgroups of PwMS in a more targeted manner. However, such dose-response relations and the moderating effects of baseline fatigue levels need to be further explored.6
Another explanation for the lack of an exercise effect is the documented compliance. In long-term and especially home-based intervention trials, achieving high compliance is always a challenge. In the current study, the compliance with the exercise regimen was quite variable. The stagnation or decline of exercise sessions per month has also been observed in other internet-based interventions with PwMS.25,26,37 A reason might be the decrease in motivation to carry out and document the exercise sessions over long study periods. Relapses and family- or work-related events also had an impact on the participants’ compliance. To effectively increase the compliance with the internet-based intervention, future developments should integrate recent theory and evidence-based approaches in the promotion of motivation and behavior change considering individual needs, preferences, motives, and perceived barriers.38,39 For example, goal setting, action and coping planning and self-monitoring are effective elements for physical activity promotion.39 Especially, self-monitoring is one of the most effective behavior change techniques in clinical populations.40 In particular, accelerometers or consumer-wearables allow patients to monitor and record their daily physical activity and are suitable in support of other behavior change techniques such as individual goal-setting or giving feedback on behavior.41 Particularly promising is an increased level of support23,26 and stronger emphasis on the social aspects of training.42,43
The results of the feasibility assessment showed that the majority of participants were very satisfied with the usability and therapeutic support of this internet-based intervention. Results are similar to the investigation of a prior study with this internet-based system.24 However, there might be a selection bias, because only those individuals who came to the last visit were assessed who might be more satisfied with the internet intervention and its components. Additionally, participants had the feeling that an internet-based approach to deliver exercise training is a useful and meaningful approach. Future research should evaluate how PwMS at a population level evaluate the acceptance and usefulness of internet-based intervention.
It is a limitation that the endurance training intensity was monitored only via the Borg scale instead of an objective heart rate monitor. However, this method was more feasible and applicable for most of the participants.
The incidence of AEs is within the range from previous studies. Accordingly, the incidence of AEs in the four-month study FIRST was 75.3%.44 Reports from observational studies with an observational period of 12 months give AE incidence rates of 35% to 60%.45,46 Noteworthy is that no participant reported any cardiovascular adverse event during the six-month study period. The proportion of relapse-free PwMS during the study period was about 90% in both groups. This result is in line with previous findings that physical exercise has no negative effects on PwMS. It suggests that a structured exercise program including strength and endurance training for PwMS under fingolimod therapy is safe and well tolerated. In this regard, the current study specifically addressed current limitations that were reported by the recent Cochrane review17such as the reporting of disease-modifying therapy, AEs and compliance with the exercise prescription.
In conclusion, consistent with previous studies, the present results suggest that physical exercise does not impose a risk on PwMS with high fatigue and/or low level of physical capacity. The beneficial effects of physical exercise on fatigue may depend on the physical capacity and or fatigue level of PwMS and require an individualized training regimen. Although internet-based interventions lack a direct therapeutic supervision, PwMS with a low aerobic capacity and/or high fatigue level can be recommended to such a home-based, combined resistance and endurance training with moderate intensities. However, this cannot be generalized to people with more severe disability. Studies that investigate the effects of different training regimens and evaluate optimal training intensities for PwMS by distinguishing between differences in physical fitness and fatigue levels are needed. Overall, considering the variety of physical activity and exercise benefits and the high variability in adherence rates, PwMS should consequently be motivated and empowered to engage in physical exercise considering the systematic integration of behavior change models and techniques. This internet-based intervention was well accepted and could facilitate the delivery of individualized, location-independent exercise training support with large sample sizes over six to 12 months. Thus, it may function as an important extension of the range of prevention or rehabilitation services. However, an analysis of the cost-effectiveness of such an intervention approach is pending.
Supplementary Material
Acknowledgement
Medical writing support was provided by Winicker Norimed GmbH and Dr Karin Eichele from mediwiz.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study and medical writing support was funded by Novartis Pharma GmbH.
Conflicts of interest
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Mathias Mäurer has received honoraria for lecturing and financial support for research from Bayer Health Care, Böhringer Ingelheim, Biogen, Genzmye/Sanofi, Merck Serono, Novartis, Roche, Talecris, and Teva; serves on a steering committee for Biogen and Novartis; and serves as a consultant for Biogen, Genzyme, and Roche. Katrin Schuh, Sabine Seibert, Monika Baier and Christian Hentschke are employees of Novartis Pharma GmbH. Alexander Tallner has received honoraria for lectures and travel grants from Novartis, Bayer Healthcare, Biogen, Merck Serono, and Teva. At the time of authorship, he was a full-time employee of the Friedrich-Alexander-Universitaet Erlangen-Nürnberg. Currently, he is employed at MedDay Pharmaceuticals GmbH. Klaus Pfeifer and René Streber from the Institute of Sport Science and Sport (University Erlangen-Nürnberg) have nothing to declare.
Supplementary material
Supplementary material is available for this article online.
References
- 1.Fisk JD, Pontefract A, Ritvo PG, et al. The impact of fatigue on patients with multiple sclerosis. Can J Neurol Sci 1994; 21: 9–14. [PubMed] [Google Scholar]
- 2.Zwibel HL. Contribution of impaired mobility and general symptoms to the burden of multiple sclerosis. Adv Ther 2009; 26: 1043–1057. [DOI] [PubMed] [Google Scholar]
- 3.Hadjimichael O, Vollmer T, Oleen-Burkey M, et al. Fatigue characteristics in multiple sclerosis: The North American Research Committee on Multiple Sclerosis (NARCOMS) survey. Health Qual Life Outcomes 2008; 6: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Multiple Sclerosis Council for Clinical Practice Guidelines. Fatigue and multiple sclerosis: Evidence-based management strategies for fatigue in multiple sclerosis. Washington, DC: Paralyzed Veterans of America, 1998. [Google Scholar]
- 5.Pittion-Vouyovitch S, Debouverie M, Guillemin F, et al. Fatigue in multiple sclerosis is related to disability, depression and quality of life. J Neurol Sci 2006; 243: 39–45. [DOI] [PubMed] [Google Scholar]
- 6.Langeskov-Christensen M, Bisson EJ, Finlayson ML, et al. Potential pathophysiological pathways that can explain the positive effects of exercise on fatigue in multiple sclerosis: A scoping review. J Neurol Sci 2017; 373: 307–320. [DOI] [PubMed] [Google Scholar]
- 7.Platta ME, Ensari I, Motl RW, et al. Effect of exercise training on fitness in multiple sclerosis: A meta-analysis. Arch Phys Med Rehabil 2016; 97: 1564–1572. [DOI] [PubMed] [Google Scholar]
- 8.Latimer-Cheung AE, Pilutti LA, Hicks AL, et al. Effects of exercise training on fitness, mobility, fatigue, and health-related quality of life among adults with multiple sclerosis: A systematic review to inform guideline development. Arch Phys Med Rehabil 2013; 94: 1800–1828.e3. [DOI] [PubMed] [Google Scholar]
- 9.Pearson M Dieberg G andSmart N.. Exercise as a therapy for improvement of walking ability in adults with multiple sclerosis: A meta-analysis. Arch Phys Med Rehabil 2015; 96: 1339–1348.e7. [DOI] [PubMed] [Google Scholar]
- 10.Kuspinar A Rodriguez AM andMayo NE.. The effects of clinical interventions on health-related quality of life in multiple sclerosis: A meta-analysis. Mult Scler 2012; 18: 1686–1704. [DOI] [PubMed] [Google Scholar]
- 11.Ensari I Motl RW andPilutti LA.. Exercise training improves depressive symptoms in people with multiple sclerosis: Results of a meta-analysis. J Psychosom Res 2014; 76: 465–471. [DOI] [PubMed] [Google Scholar]
- 12.Dalgas U, Stenager E, Sloth M, et al. The effect of exercise on depressive symptoms in multiple sclerosis based on a meta-analysis and critical review of the literature. Eur J Neurol 2015; 22: 443–e34. [DOI] [PubMed] [Google Scholar]
- 13.Pilutti LA, Platta ME, Motl RW, et al. The safety of exercise training in multiple sclerosis: A systematic review. J Neurol Sci 2014; 343: 3–7. [DOI] [PubMed] [Google Scholar]
- 14.Dalgas U Stenager E andIngemann-Hansen T.. Multiple sclerosis and physical exercise: Recommendations for the application of resistance-, endurance- and combined training. Mult Scler 2008; 14: 35–53. [DOI] [PubMed] [Google Scholar]
- 15.Tallner A, Waschbisch A, Wenny I, et al. Multiple sclerosis relapses are not associated with exercise. Mult Scler 2012; 18: 232–235. [DOI] [PubMed] [Google Scholar]
- 16.Dalgas U andStenager E.. Exercise and disease progression in multiple sclerosis: Can exercise slow down the progression of multiple sclerosis? Ther Adv Neurol Disord 2012; 5: 81–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heine M, van de Port I, Rietberg MB, et al. Exercise therapy for fatigue in multiple sclerosis. Cochrane Database Syst Rev 2015; CD009956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pilutti LA, Greenlee TA, Motl RW, et al. Effects of exercise training on fatigue in multiple sclerosis: A meta-analysis. Psychosom Med 2013; 75: 575–580. [DOI] [PubMed] [Google Scholar]
- 19.Andreasen AK Stenager E andDalgas U.. The effect of exercise therapy on fatigue in multiple sclerosis. Mult Scler 2011; 17: 1041–1054. [DOI] [PubMed] [Google Scholar]
- 20.Rimmer JH. Getting beyond the plateau: Bridging the gap between rehabilitation and community-based exercise. PM R 2012; 4: 857–861. [DOI] [PubMed] [Google Scholar]
- 21.Aalbers T Baars MA andRikkert MG.. Characteristics of effective Internet-mediated interventions to change lifestyle in people aged 50 and older: A systematic review. Ageing Res Rev 2011; 10: 487–497. [DOI] [PubMed] [Google Scholar]
- 22.Davies CA, Spence JC, Vandelanotte C, et al. Meta-analysis of internet-delivered interventions to increase physical activity levels. Int J Behav Nutr Phys Act 2012; 9: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Webb TL, Joseph J, Yardley L, et al. Using the internet to promote health behavior change: A systematic review and meta-analysis of the impact of theoretical basis, use of behavior change techniques, and mode of delivery on efficacy. J Med Internet Res 2010; 12: e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tallner A, Tzschoppe R, Peter S, et al. Internetgestützte Bewegungsförderung bei Personen mit Multipler Sklerose. Neurologie & Rehabilitation 2013; 19: 35–46. [Google Scholar]
- 25.Tallner A, Streber R, Hentschke C, et al. Internet-supported physical exercise training for persons with multiple sclerosis—A randomised, controlled study. Int J Mol Sci 2016; 17: 1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Motl RW, Dlugonski D, Wojcicki TR, et al. Internet intervention for increasing physical activity in persons with multiple sclerosis. Mult Scler 2011; 17: 116–128. [DOI] [PubMed] [Google Scholar]
- 27.Dlugonski D, Motl RW, Mohr DC, et al. Internet-delivered behavioral intervention to increase physical activity in persons with multiple sclerosis: Sustainability and secondary outcomes. Psychol Health Med 2012; 17: 636–651. [DOI] [PubMed] [Google Scholar]
- 28.Romberg A, Virtanen A, Ruutiainen J, et al. Effects of a 6-month exercise program on patients with multiple sclerosis: A randomized study. Neurology 2004; 63: 2034–2038. [DOI] [PubMed] [Google Scholar]
- 29.Krupp LB. Fatigue in multiple sclerosis: Definition, pathophysiology and treatment. CNS Drugs 2003; 17: 225–234. [DOI] [PubMed] [Google Scholar]
- 30.Meca-Lallana J, Hernández L, Caminero AB, et al. Fatigue improvement after switching multiple sclerosis treatment from interferon-β to glatiramer acetate in clinical practice. Eur Neurol 2016; 76: 40–47. [DOI] [PubMed] [Google Scholar]
- 31.Fox E, Edwards K, Burch G, et al. Outcomes of switching directly to oral fingolimod from injectable therapies: Results of the randomized, open-label, multicenter, Evaluate Patient OutComes (EPOC) study in relapsing multiple sclerosis. Mult Scler Relat Disord 2014; 3: 607–619. [DOI] [PubMed] [Google Scholar]
- 32.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982; 14: 377–381. [PubMed] [Google Scholar]
- 33.Fisk JD, Ritvo PG, Ross L, et al. Measuring the functional impact of fatigue: Initial validation of the Fatigue Impact Scale. Clin Infect Dis 1994; 18 (Suppl 1): S79–S83. [DOI] [PubMed] [Google Scholar]
- 34.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An Expanded Disability Status Scale (EDSS). Neurology 1983; 33: 1444–1452. [DOI] [PubMed] [Google Scholar]
- 35.Fischer JS, Rudick RA, Cutter GR, et al. The Multiple Sclerosis Functional Composite Measure (MSFC): An integrated approach to MS clinical outcome assessment. National MS Society Clinical Outcomes Assessment Task Force. Mult Scler 1999; 5: 244–250. [DOI] [PubMed] [Google Scholar]
- 36.Rudick RA Cutter G andReingold S.. The Multiple Sclerosis Functional Composite: A new clinical outcome measure for multiple sderosis [sic] trials. Mult Scler 2002; 8: 359–365. [DOI] [PubMed] [Google Scholar]
- 37.Dlugonski D Motl RW andMcAuley E.. Increasing physical activity in multiple sclerosis: Replicating Internet intervention effects using objective and self-report outcomes. J Rehabil Res Dev 2011; 48: 1129–1136. [DOI] [PubMed] [Google Scholar]
- 38.Streber R Peters S andPfeifer K.. Systematic review of correlates and determinants of physical activity in persons with multiple sclerosis. Arch Phys Med Rehabil 2016; 97: 633–645.e29. [DOI] [PubMed] [Google Scholar]
- 39.Geidl W Semrau J andPfeifer K.. Health behaviour change theories: Contributions to an ICF-based behavioural exercise therapy for individuals with chronic diseases. Disabil Rehabil 2014; 36: 2091–2100. [DOI] [PubMed] [Google Scholar]
- 40.Conn VS, Hafdahl AR, Brown SA, et al. Meta-analysis of patient education interventions to increase physical activity among chronically ill adults. Patient Educ Couns 2008; 70: 157–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koring M, Parschau L, Lange D, et al. Preparing for physical activity: Pedometer acquisition as a self-regulatory strategy. Appl Psychol Health Well Being 2013; 5: 136–147. [DOI] [PubMed] [Google Scholar]
- 42.Maher CA, Lewis LK, Ferrar K, et al. Are health behavior change interventions that use online social networks effective? A systematic review. J Med Internet Res 2014; 16: e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Learmonth YC, Marshall-McKenna R, Paul L, et al. A qualitative exploration of the impact of a 12-week group exercise class for those moderately affected with multiple sclerosis. Disabil Rehabil 2013; 35: 81–88. [DOI] [PubMed] [Google Scholar]
- 44.Gold R, Comi G, Palace J, et al. Assessment of cardiac safety during fingolimod treatment initiation in a real-world relapsing multiple sclerosis population: A phase 3b, open-label study. J Neurol 2014; 261: 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tichá V, Kodým R, Počiková Z, et al. Real-world outcomes in fingolimod-treated patients with multiple sclerosis in the Czech Republic: Results from the 12-month GOLEMS study. Clin Drug Investig 2017; 37: 175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Achiron A, Aref H, Inshasi J, et al. Effectiveness, safety and health-related quality of life of multiple sclerosis patients treated with fingolimod: Results from a 12-month, real-world, observational PERFORMS study in the Middle East. BMC Neurol 2017; 17: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.