Fig. 1.
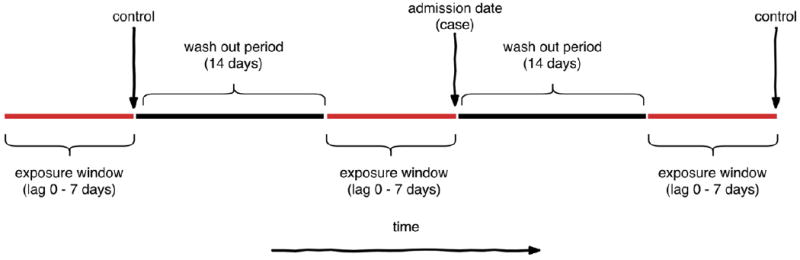
Case-crossover study design. Caseswere defined as ED visit dates and were considered to be exposed to a CSO event if the ED visit occurredwithin 7 days and 500mof a CSO event. Two bi-directional control periods for each casewere defined as 14 days before and after the case period. For each outcome, lag periods of 0 to 7 dayswere examined in order to consider varying time periods between CSO events and disease onset considering variation in exposure routes and incubation periods.
