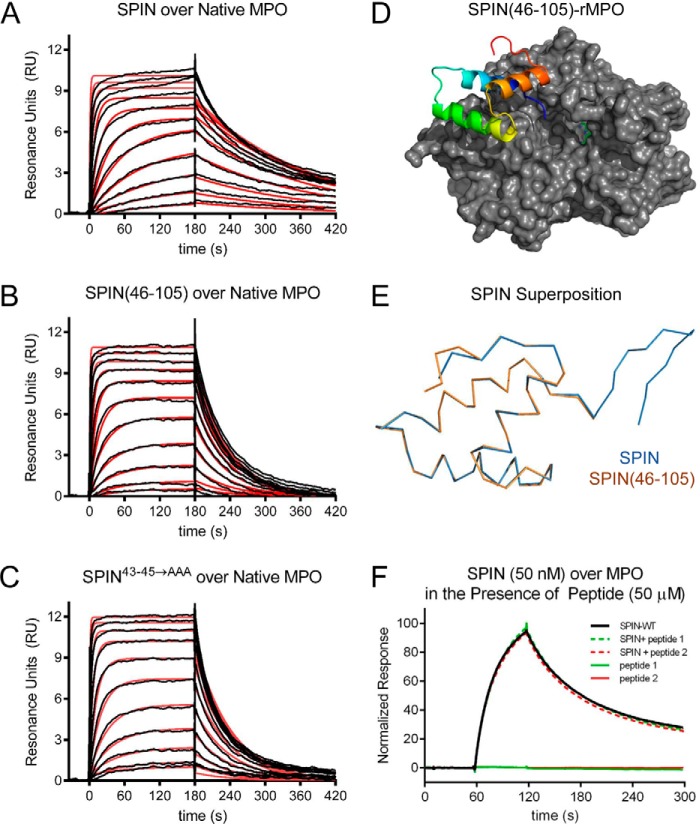
Figure 2.
N-terminal region of SPIN is dispensable for binding to MPO. The ability of SPIN proteins and SPIN-derived peptides to bind MPO was examined using a combination of biochemical and structural methods. A 2-fold dilution series of SPIN proteins ranging from 1.35 to 2000 nm was injected over a biosensor surface prepared from randomly immobilized MPO. Representative sensorgram series are shown for native MPO binding to full-length SPIN (A), SPIN(46–105) (B), and SPIN43–45→AAA (C). Additional curve fitting and analysis parameters are presented in Table 1. D, representation of a 2.3 Å resolution co-crystal structure of SPIN(46–105) bound to a recombinant form of human MPO. SPIN(46–105) is depicted as a ribbon diagram and is colored with its N terminus in blue and its C terminus in red, and MPO is rendered as a gray surface. The location of the MPO active-site heme is indicated by a green ball-and-stick for the purposes of reference. E, superposition of the MPO-bound forms of SPIN(46–105) and full-length SPIN, as judged by X-ray crystallography. Proteins are depicted as wire diagrams, where the N-terminal β-hairpin of full-length SPIN is shown at the top right. F, MPO-binding properties of two different synthetic peptides corresponding to the SPIN N-terminal region were assessed by SPR. Peptide binding was investigated using both a direct binding approach (single injection at 50 μm) and through a competition format where peptides were co-injected along with SPIN (50 nm). Neither approach showed any evidence for binding between these peptides and MPO. A representative sensorgram series is shown.