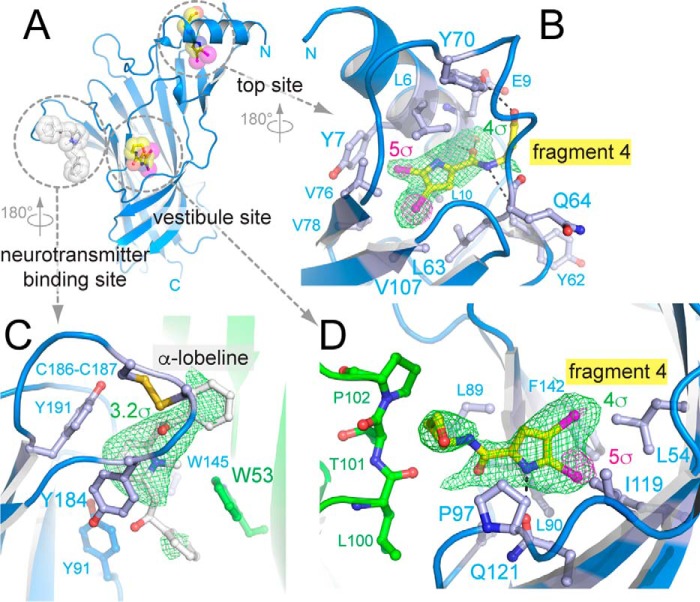
Figure 2.
X-ray crystal structure of α7-AChBPVS in complex with α-lobeline and allosteric binder fragment 4. A, schematic representation of a single α7-AChBPVS subunit as seen toward the vestibule site. α-Lobeline is bound to the neurotransmitter-binding site and is shown in white transparent sphere and stick representation. Fragment 4 is bound to two different allosteric sites, the vestibule site and the top site and is shown in yellow (carbon), blue (nitrogen), red (oxygen), and magenta (bromine). B, detailed view of the amino acid interactions formed by fragment 4 at the top site. The green mesh is the 5-fold averaged simple difference density at a contour level of 4σ, the magenta mesh is the anomalous difference density at 5σ. C, detailed view of amino acid interactions formed by α-lobeline at the neurotransmitter binding site. The principal subunit is shown in blue, the complementary subunit in green. The highly conserved aromatic side chains are shown as ball and sticks. The green mesh is the 5-fold averaged simple difference density at 3.2σ. D, detailed view of amino acid interactions formed by fragment 4 at the vestibule site. Most interactions are intrasubunit interactions (blue), whereas 3 residues come from a neighboring subunit (green). The green mesh is the 5-fold averaged simple difference density at a contour level of 4σ, the magenta mesh is the anomalous difference density at 5σ.