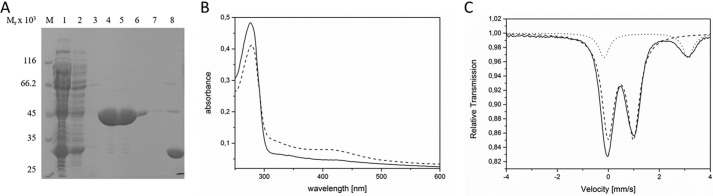
Figure 2.
Spectroscopic analyses of the HemW-bound [4Fe–4S]2+ cluster. A, SDS-PAGE analysis of the purification of recombinant HemW from E. coli. Recombinant HemW in E. coli cell-free lysates was loaded onto glutathione-Sepharose. The immobilized GST-HemW fusion was digested by PreScission protease, and released HemW was eluted. Resulting fractions were separated via SDS-PAGE, and proteins were visualized using Coomassie Blue staining. In lane 1, total cellular extract was loaded; lanes 2 and 3 show the washing steps; lanes 4–7 show the purified HemW protein; and lane 8 shows the eluate of the cleaved GST tag. A marker mixture with proteins of known relative molecular weights is shown in lane M. B, UV-visible spectra of 10 μm HemW before (solid line) and after (dashed line) reconstitution of the iron–sulfur cluster. C, zero-field Mössbauer spectrum of purified HemW (0.55 mm) recorded at 80.00 K. The solid line represents a fit of the experimental data (dots) with two Lorentzian quadrupole doublets (dashed and dotted lines). The analysis revealed a dominant component (83% of the total intensity) with an isomer shift (δ) of 0.49 mm/s and a quadrupole splitting parameter (ΔEQ) of 1.00 mm/s and second quadrupole doublet (17% of the total intensity) with δ = 1.48 mm/s and ΔEQ = 3.30 mm/s (dotted line). The high isomer shift of the latter excludes an origin from [Fe–S] clusters but reveals high-spin Fe(II) sites with six hard oxygen or nitrogen ligands; the component is therefore assigned to adventitiously bound Fe(II) in the protein, presumably remaining from the reconstitution procedure.