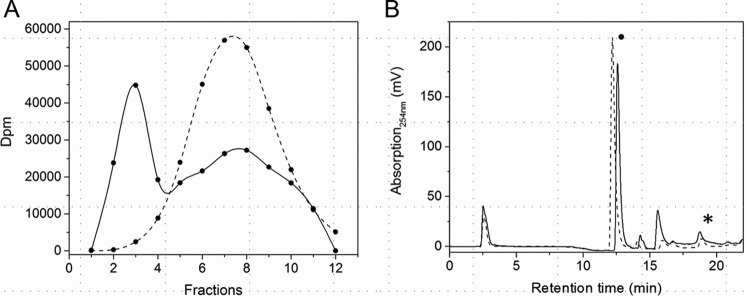
Figure 5.
SAM binding and SAM cleavage by HemW. A, SAM binding assay. 100 μm HemW was incubated with 0.5 μCi of [14C]SAM and fractionated via a desalting column. The radioactive fractions were analyzed using liquid scintillation counting. Solid line, HemW + [14C]SAM; dashed line, BSA + [14C]SAM. B, the SAM cleavage assays were performed for 25 μm E. coli HemW (solid line) supplemented with heme, 25 μm E. coli HemN without substrate (dashed line), and 25 μm E. coli HemN with its substrate coproporphyrinogen III (dotted line). After addition of 0.6 mm dithionite as a potential electron donor, 0.6 mm SAM was added, and the mixture was incubated. The reaction was stopped with formic acid. Samples were chromatographically separated on a Hypercarb column with appropriate marker substances. SAM (indicated with a dot) and formed 5′-deoxyadenosine (indicated with a star) were detected at 254 nm. Background controls without protein or BSA did not yield the 5′-deoxyadenosine–specific peak. HemN in the presence of substrate revealed full SAM cleavage. Without substrate, 5% residual SAM cleavage was observed for HemN. HemW with heme revealed comparable residual SAM cleavage activity.