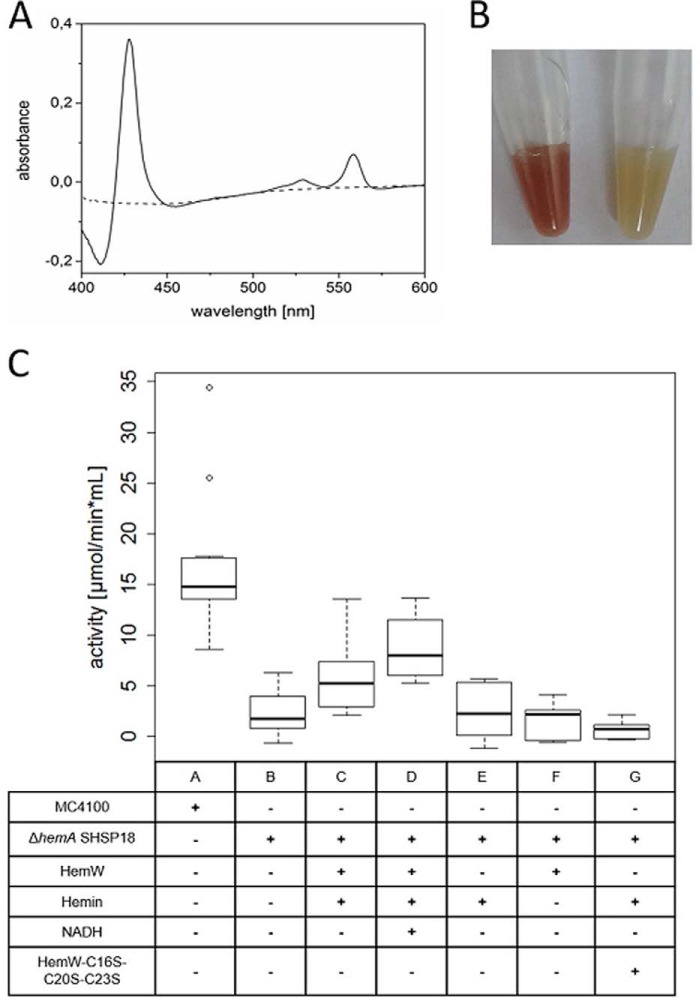
Figure 9.
HemW-mediated heme transfer to the nitrate oxidoreductase NarGHI. A, comparative spectroscopic analysis of prepared membrane vesicles from E. coli wildtype MC4100 and the corresponding E. coli ΔhemA mutant. The recorded absorption spectrum at around A425 indicated the absence of bound heme cofactor in NarGHI produced by E. coli ΔhemA mutant (dashed line) in contrast to the spectrum recorded for the identical membrane vesicle preparation from wildtype E. coli. B, the decolorization of the prepared membrane vesicles (left tube, wildtype; right tube, ΔhemA mutant) due to the depletion of heme is optically visible. C, enzyme assays were performed with membrane vesicles isolated from E. coli MC4100/pVA700 overexpressing narGHJI (labeled MC4100) and membrane vesicles with overproduced heme-depleted nitrate oxidoreductase isolated from E. coli ΔhemA/pVA700 (labeled ΔhemA SHSP18). The heme-depleted nitrate oxidoreductase was incubated with HemW-heme (C), HemW-heme + NADH (D), and [4Fe–4S] clusterless HemW-C16S/C20S/C23S–heme (G) and as negative controls solely with heme (E), HemW (F), or HemW and NADH (H), respectively. 20 mm 2-methyl-1,4-naphtoquinol (menadiol) served as electron donor, 2 mm nitrate served as electron acceptor, and 5 mm NADH and 1.5 mm free heme were used where indicated. The range between −1 and 18 μmol/min × ml is shown. Error bars represent S.D.