Figure 6.
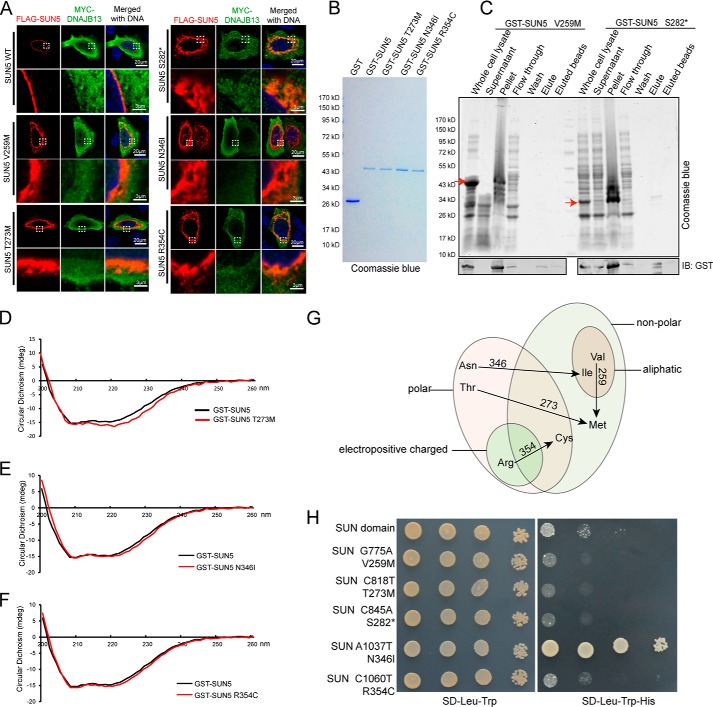
Mutations in the SUN domain affected protein secondary structures, localization, and interaction with DNAJB13. A, mutations in the SUN domain affected the distribution of full-length SUN5 in mammalian cells and recruitment of DNAJB13. WT–FLAG–SUN5 or its variants, together with MYC–DNAJB13, were co-transfected into HeLa cells. After a 24-h incubation, the transfected cells were immunostained with anti-FLAG (red) and anti-MYC (green) antibodies. The nuclear DNA was stained with DAPI (blue). WT–FLAG–SUN5 localized to the NE, and DNAJB13 was partially recruited to the NE. FLAG–SUN5 V259M, T273M, S282*, N346I, and R354C showed abnormal localization and could not recruit DNAJB13 to the NE. The boxed areas have been enlarged in the lower row. B, GST–SUN5 and its variants. GST–SUN5 and its variant proteins were expressed in E. coli and purified by GST–beads. They were separated using SDS-PAGE and stained with Coomassie Blue. C, the mutations V259M and S282* led to protein precipitation during in vitro purification. The samples were separated by SDS-PAGE and stained with Coomassie Blue. D–F, the secondary structure of the GST–SUN5 protein was affected by incorporated mutations T273M, N346I, and R354C, as revealed by CD analysis. G, all of the SUN domain mutations caused striking changes to amino acid properties. H, most mutations in the SUN domain impaired SUN5–DNAJB13 interaction as characterized by the Y2H system. WT SUN (SUN domain of SUN5) or its variants were co-transformed with DNAJB13 in the Y2H assay, and yeast cells were diluted by 101, 102, and 103 and then spotted onto control (SD-Leu-Trp) and selective (SD-Leu-Trp-His) plates.