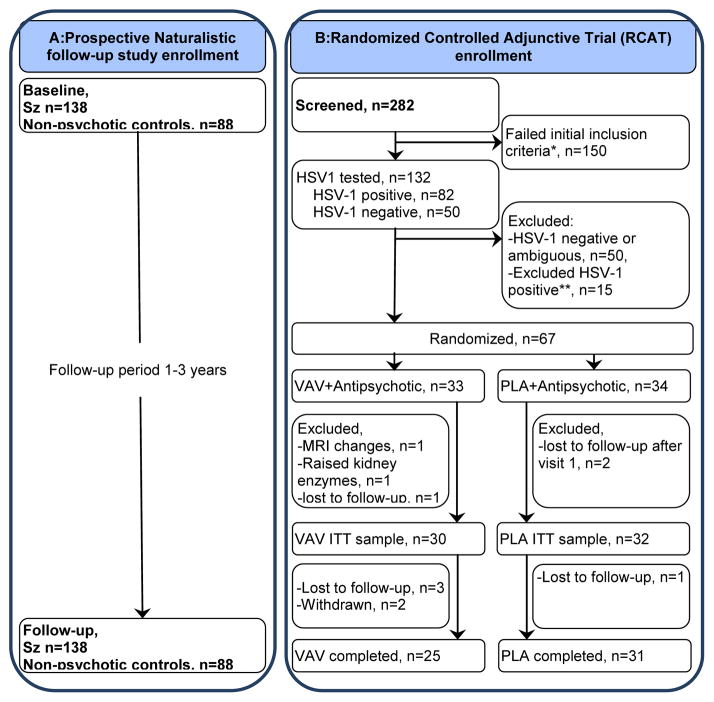
Figure 1. Flow of participants in Both Studies.
A: Prospective Naturalistic follow-up study enrollment B: Randomized Controlled Adjunctive Trial (RCAT) enrollment D
Treatment trial exclusions: aReasons for exclusion after screening: symptomatic n=5; transport problems n=8; refused, n=8; could not be contacted after screening, n=36; medical illness, n=4; comorbidity, n=6; illness >7yrs, n=16; age <18 years, n=1; age >50years, n=14; not stable on medication, n=18; illness <one year, n=7; other, n=35. bReasons for exclusion after HSV-1 assays: Raised liver enzymes, N=2, Raised uric acid levels, N=2, Refused, N=4, Lost contact, N=3, Diagnosed with gall bladder stones, N=1, Dystonia, N=1, Worsened symptoms, N=1, Age above 50, N=1