Abstract
Mediator complex, a conserved multi-protein, is necessary for controlling RNA polymerase II (Pol II) transcription in eukaryotes. Given little is known about them in tomato, a tomato Mediator subunit 18 gene was isolated and named SlMED18. To further explore the function of SlMED18, the transgenic tomato plants targeting SlMED18 by RNAi-mediated gene silencing were generated. The SlMED18-RNAi lines exhibited multiple developmental defects, including smaller size and slower growth rate of plant and significantly smaller compound leaves. The contents of endogenous bioactive GA3 in SlMED18 silenced lines were slightly less than that in wild type. Furthermore, qRT-PCR analysis indicated that expression of gibberellins biosynthesis genes such as SlGACPS and SlGA20x2, auxin transport genes (PIN1, PIN4, LAX1 and LAX2) and several key regulators, KNOX1, KNOX2, PHAN and LANCEOLATE(LA), which involved in the leaf morphogenesis were significantly down-regulated in SlMED18-RNAi lines. These results illustrated that SlMED18 plays an essential role in regulating plant internode elongation and leaf expansion in tomato plants and it acts as a key positive regulator of gibberellins biosynthesis and signal transduction as well as auxin proper transport signalling. These findings are the basis for understanding the function of the individual Mediator subunits in tomato.
Introduction
Plant growth and development, including vegetative growth and reproductive growth, is a multiphase process that requires a tight coordination among molecular, biochemical and structural elements1. The vegetative growth includes the development of root, stem and leaf, the reproductive growth includes flower, fruit and seed development and the vegetative growth is necessary for providing the essential nutrients for the reproductive growth. Plants usually grow vegetatively at early developmental stage then translate to the reproductive growth stage2. In the vegetative growth, the roots provide water and other nutrients to the plant, and the growth of the stem provides the possibility for the plant to produce more leaves, while the development and growth of the leaves provide more nutrients and energy for the reproductive growth of the plant.
The regulation of plant gene expression is necessary for the normal growth and development of plants, which is a complex and accurate network system that generally includes both transcription and translation of a series of genes. Transcription in eukaryotic organism is an intricate and extremely orchestrated process, through RNA Pol II assisted by a number of transcriptional regulators. The transcriptional regulators include transcriptional activators, various general transcription factors (GTFs), and a series of transcription cofactors3,4. Several transcription factor families, including MADS-box, GRAS, and MYB have been characterized for their regulatory roles in plant vegetative development5–7. In addition, Mediator, a multi-subunit complex, is one cofactor that promotes transcription initiation as the bridges between transcription activators bounding to regulatory upstream promoter or enhances DNA elements and the RNA Pol II general transcriptional machinery at the essential promoter8–11. Nowadays, Mediator complex has emerged as possibly the most essential section for regulating Pol II transcription in eukaryotes.
Mediator complex was first identified in Saccharomyces cerevisiae and structural studies showed that the 21 yeast Mediator subunits have been divided into four submodules, the head, middle, tail, and kinase modules12. Moreover, the modular architecture and subunit composition of Mediator complex is evolutionarily conserved in eukaryotes13. In plants, more than 30 different Mediator subunits have been shown that are part of the Mediator complex in different organisms. In the past, the studies of Mediator complex have been focused on yeasts and metazoans. Until 2007, more than a decade after its discovery in S. cerevisiae, Mediator complex was successfully purified from plants14. Since then, some plants Mediators and the physiological functions of several subunits began to be revealed. The discovery of the Mediator complex in Arabidopsis thaliana indicated the Mediator complex subunits possess their own specific function within the whole complex, and already, a majority of Mediator complex subunits have been reported to have important regulatory roles. In Arabidopsis thaliana, AtMED12 and AtMED13, referred to as GRAND CENTRAL (GCT) and CENTER CITY (CCT), effect various aspects of plant development including embryo pattern formation, developmental timing and flowering and floral morphogenesis15,16. The genetic and physiological study suggested that AtMED25/ PHYTOCHROME AND FLOWERING TIME 1 (PFT1) are implicated in regulating JA-triggered gene expression and effect flowering under suboptimal light conditions14,17–21. AtMED14, which was originally described as STRUWWELPETER (SWP), affects cell proliferation and shoot meristem development. AtMED20a, AtMED18, AtMED8, and AtMED17 were known to implicated in non-coding RNA production22,23. AtMED14, AtMED15, and AtMED16 have recently been speculated to be involved in defense signalling24–26. In another study, Arabidopsis Med32 played a role in root development and senescence. Although there are some studies on the Mediator function in model plant which made some meaningful progress, the roles of plant mediator in RNA transcription remain to be further elucidated on account of the lack of relevant mutant gene material.
MED18 (Mediator subunit 18 gene) is one of the Mediator complex subunits genes. Structural studies illustrated that MED18 is located in the movable jaw of the head module structure and resembles the head of a crocodile with one limb13. Recently several studies about MED18 in Arabidopsis thaliana have been reported that AtMED18 was involved in regulating multiple plant physiological processes, including plant immunity, abscisic acid (ABA) responses, flowering time and floral organ identity through interactions with distinct transcription factors27–30.
Solanum lycopersicum is a kind of widely grown economic crop, which not only has high agricultural economic value but also is an important model plant for studying plants development. To date, although Mediator complex plays a critical role in promoting the transcription of genes, little is known about them in tomato. In this study, a member of mediator family, named Mediator subunit 18 (SlMED18), was isolated form tomato. To further explore the function of SlMED18 in plant growth and development, we created transgenic tomato plants using S. lycopersicon Mill. cv. Ailsa Craig++ as wild type tomato by RNA interference. Down-regulation of SlMED18 restricted internode elongation and leaf expansion, producing dwarf plants and smaller leaves than wild type. The molecular and cellular levels investigation of SlMED18-RNAi lines indicated that SlMED18 plays an important role in regulating the development of leaf and stem. This research enhanced our knowledge about the roles of SlMED18 in tomato developmental processes.
Results
SlMED18 isolation and expression pattern analysis
In this study, a Solanum lycopersicum Mediator of RNA polymerase II transcription subunit 18 gene, named SlMED18, was isolated from wild type tomato leaves with specific primers (Supplementary Table S1) based on a cDNA clone (GenBank accession no. XM_010323502.2). Sequencing validation indicated that the correct gene sequence was obtained and sequence analysis showed that SlMED18 contains an open reading frame (ORF) of 651 bp and encodes 216 amino acid residues.
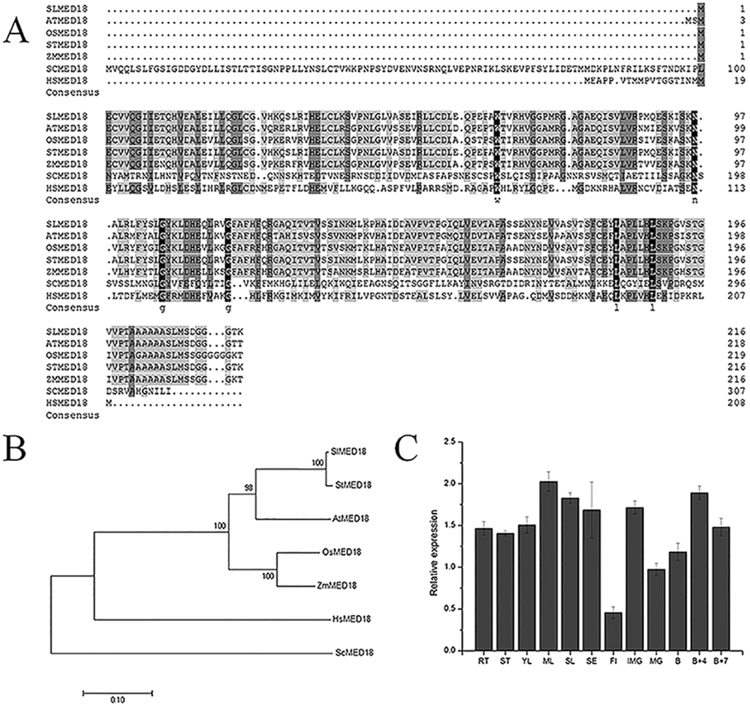
In addition, keyword searches and BLAST searches were performed against the Arabidopsis thaliana Mediator complex subunit gene sequences. To dissect the evolutionary relationships of MED18, the MED18 sequences of tomato, Zea mays(Zm), Solanum tuberosum (St), S. cerevisiae, Human (Hs), the dicot model plant Arabidopsis thaliana (At) and the monocot model plant Oryza sativa (Os)were used to conduct multiple sequence alignment and an original tree was constructed. Phylogenic analysis implied that sequences are weakly conserved between S. cerevisiae, Human (Hs) and plants, but they are conserved across the plant kingdom (Fig. 1A,B).
Figure 1.
Evolutionary relationships and transcriptional pattern of SlMED18 in wild type tomato. (A) Multiple sequence alignment of MED18 protein in S. lycopersicum (Sl), Arabidopsis thaliana (At), Oryza sativa (Os), Zea mays (Zm), Solanum tuberosum (St), S. cerevisiae (Sc) and Human (Hs). The numbers on the right indicate the positions of amino acid residues. The identical amino acids are shaded in black, and similar amino acids are shaded in gray. (B) Phylogenetic relationship of MED18 in tomato and other species. The tree was constructed from a complete alignment of 7 MED18 amino acid sequences by MEGA7, using the Neighbor-Joining method. And the evolutionary distances were computed by the p-distance method. (C) Transcriptional pattern of SlMED18 in wild type tomato. RT, root; ST stem; YL, young leaf; ML mature leaf; SL, senescent leaf; SE, sepal of flower in anthesis; FI, flower; IMG, immature green fruit; MG, mature green fruit; B, breaker fruit; B + 4, 4 days after breaker fruit; B + 7, 7 days after breaker fruit. Each value represents the mean ± SD of three replicates.
As we all know that the tissue specificity of gene expression may be correlated with specific biological functions. So the real-time PCR analysis was conducted to clarify the expression profile of SlMED18 in various tissues, which consist of roots, stems, leaves, flowers and fruits at different developmental stages (Fig. 1C). The results showed that SlMED18 expressed abundantly in all the organizations we examined, which was consistent with pre-expression pattern (Supplementary Fig. S1). The bar graph of expression profile in various tomato tissues was obtained from Tomato lab website. These results indicated that SlMED18 may have essential roles in multiple tomato plant growth and development process.
Repression of SlMED18 causes plant developmental defects
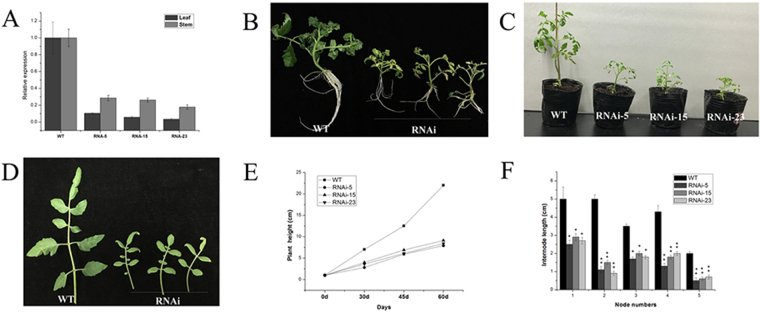
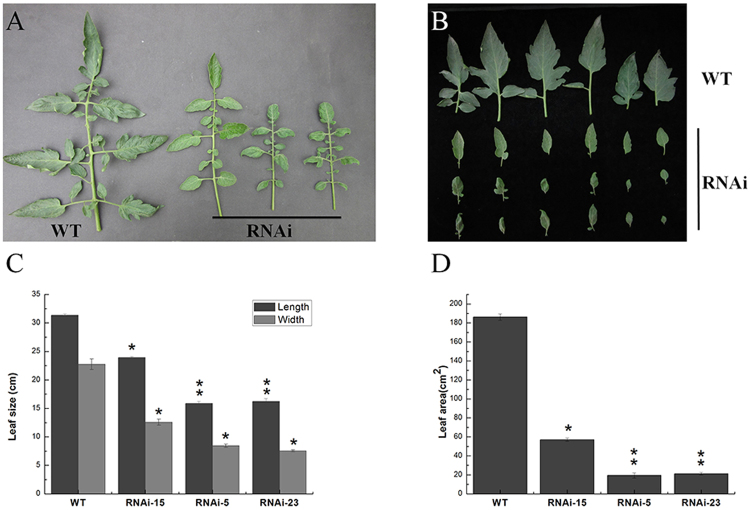
To further investigate the biological functions of SlMED18 in tomato, an RNAi expression vector targeting SlMED18 gene was created and transformed into tomato (Solanum lycopersicum ‘Ailsa Craig’ AC++) via Agrobacterium tumefactions mediated transformation. 25 independent transgenic lines were confirmed by PCR using primers of NPT II (Supplementary Table S1), then their total RNAs were extracted from young leaves to investigate the relative expression of SlMED18, respectively. qRT- PCR data showed that the three transgenic lines (RNAi 5, RNAi 15 and RNAi 23), compared with the control, displayed 90%, 93% and 96% reduction in SlMED18 mRNA levels, respectively (Fig. 2A). Later these three transgenic lines were chosen and used for subsequent experiments. Compared with wild type plants, the SlMED18-RNAi lines displayed shorter and thinner stem with significantly smaller compound leaf (Fig. 2B–D and Supplementary Fig. S2A–C). The height of 60-days-old wild type plant was about 22.5 cm, while the three transgenic lines (RNAi 5, RNAi 15 and RNAi 23) exhibited a lower growth rate and had 9.1, 8.8 and 8.0 cm plant height. Thus, the RNAi 5, RNAi 15 and RNAi 23 plants were 59.56%, 60.9% and 64.34% shorter than the control, respectively (Fig. 2E). Furthermore, the internode lengths of 60-days-old wild type and SlMED18-RNAi plant from the first to the fifth node were measured, the result showed that every corresponding internode in SlMED18-RNAi transgenic tomato plants was reduced remarkably compared with the wild type (Fig. 2F). Morphology observation displayed that the stems of SlMED18-RNAi lines were thinner than the wild type (Fig. S2A,C), and the leaves were significantly smaller (Fig. 3A,B). The length, width as well as area of mature compound leaves was significantly less than wild type (Fig. 3C,D).
Figure 2.
Repression of SlMED18 causes plant developmental defects. (A) The expression levels of SlMED18 in three SlMED18-RNAi transgenic lines (RNAi-5, RNAi-15 and RNAi-23) and wild type plants. We collected young leaves and stems of the plants as materials detected by real-time qPCR and normalized the expression data of wild type plants to 1. (B,C) The SlMED18-RNAi plants (RNAi-5, RNAi-15 and RNAi-23) were exhibited multiple developmental defects. 30-day old plants rooted on the MS culture medium in culture bottle (B) and then transplanted them into pots to cultivate 30 days (C). (D) Phenotypes of leaves in SlMED18-RNAi lines. The compound leaves were collected from 60-day-old wild type and SlMED18-RNAi lines at the same nodes. (E) The growth rate of wild type and SlMED18-RNAi plants. We measured the height of plants in 0-day old, 30-day old, 45-day old and 60 day old. (F) Internode length of the first to the fifth node from 60-days-old wild type and SlMED18-RNAi lines.
Figure 3.
Altered leaf morphology in SlMED18-RNAi tomato plants. The compound leaves (A) and the main leaflets (B) were collected from the same node (the fifth node) of 60-days old wild type and SlMED18-RNAi tomato plants. The maximum length, maximum width (C), and the area of leaves (D) compered between wild type and SlMED18-RNAi tomato plants. Error bar indicates means ± SD (n = 8). *P < 0.05 and **P < 0.01 according to t-test.
Transgenic plants have smaller leaf cells
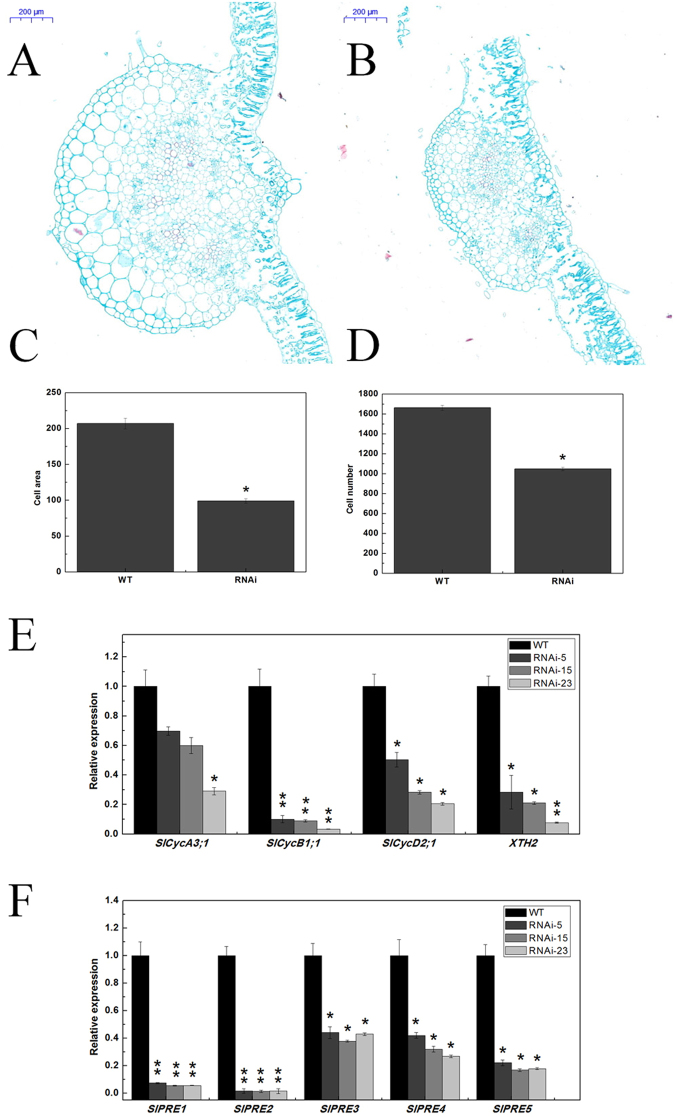
Given the differences of leaves between transgenic lines and the wild type, an anatomical investigation of sections of leaf was performed to investigate whether this phenotype was associated with a decrease in the cell size or in the cell number. The cellular analysis showed that the size of leaf cell in the SlMED18-RNAi lines was dissimilar from those in the wild type at the same stage of tomato plant development (Fig. 4A–B). The cellular data indicate that cell size of the SlMED18-RNAi leaf was significantly smaller and the cell number of leaf vein was notably less, compared with those in the wild type (Fig. 4C and D). The results suggested that the smaller and narrower leaves might due to both the reduction of leaves cell size and cell number.
Figure 4.
Anatomical analyses of leaf between wild-type and the SlMED18-RNAi lines. (A and B) The lead of wild type (A) and SMED18-RNAi plant (B) were prepared transverse sections. Bars = 200 μm. (C and D) The estimated cell area (μm2) (C) and cell number of leaf vein (D) were measured and compared between transgenic and wild type at the same position in both cases. Data are shown as the mean ± SE. (E and F) Expression of several genes associated with cell expansion and cell cycle genes (E) SlCycA3;1, SlCycB1;1, SlCycD2;1, SlXTH2; (F) SlPRE1, SlPRE2, SlPRE3, SlPRE4 in leaf.
To explore the molecular mechanism of smaller leaf of transgenic plants, some genes associated with cell expansion and cell cycle genes, such as SlCycA3;1, SlCycB1;1, SlCycD2;1, SlXTH2, SlPRE1, SlPRE2, SlPRE3, SlPRE4 and SlPRE5 were examined in leaf. As shown in Fig. 4E, the representative cell cycle regulatory genes, SlCycA3;1, SlCycB1;1 and SlCycD2;131, and the biogenesis and modification of the cell wall components related genes, SlXTH232, were down-regulated in SlMED18-RNAi lines. Moreover, the transcript levels of SlPRE1, SlPRE2, SlPRE3, SlPRE4 and SlPRE5, which involved in cell division and expansion33, were markedly decreased in SlMED18-RNAi lines (Fig. 4F).
SlMED18-RNAi plants have decreased endogenous bioactive Gibberellins (GAs) contents
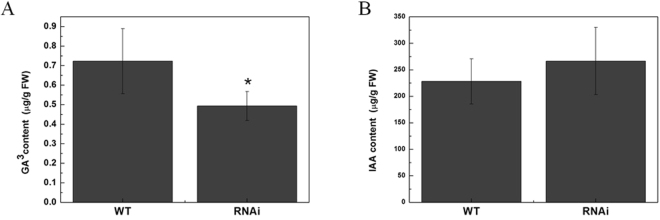
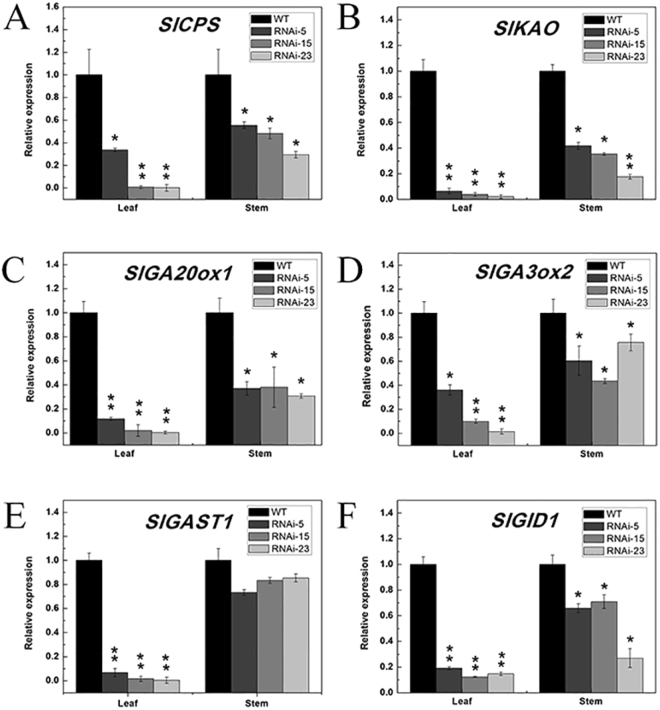
It has been reported that the plant hormone GAs is involved in internode elongation as well as other plant developmental processes. To date, a lot of genes encoding GAs biosynthetic or signalling transduction genes have been characterized as being associated with this process34,35. As the SlMED18-RNAi plants were dramatically shorter than the wild type (Fig. 2C), the contents of endogenous bioactive GA3 in the stems of wild type and SlMED18-RNAi lines were measured. The results showed that endogenous GA3 in transgenic plants were significant less than that of wild type (Fig. 5A), indicating that the dwarfism of SlMED18-RNAi plants is due to the deficiency of endogenous GAs. Therefore, we further detected some genes involved in the GAs biosynthesis and signal transduction. The transcript accumulation of two genes, tomato ent-copalyl diphosphate synthase (SlCPS) and tomato ent-kaurenoic acid oxidase (SlKAO), which involved in the GA biosynthetic pathway were markedly reduced in stems and leaves of SlMED18-RNAi lines (Fig. 6A,B). In many plant species, GA20oxs are the core GA biosynthetic enzymes that determine the GAs concentration and GA3oxs catalyze the final step to produce bioactive GAs (GA1, GA3, and GA4)36. In our study, SlGA20ox1 and SlGA3ox2 were dramatically down-regulated in leaves and stems of SlMED18-RNAi lines compared with the wild type (Fig. 6C,D). To further assess whether SlMED18 affects GA signal transduction factors, we also determined the expression level of SlGAST1 (tomato gibberellin-stimulated transcripts 1), a tomato GA-responsive downstream gene, as well as a GA receptor SlGID1-A (tomato GA INSENSITIVE DWARF1-A)37–39. The results displayed that SlGAST1 and SlGID1-A were significantly down-regulated in leaves of SlMED18-RNAi plants but not drastically decreased in stems of transgenic plants (Fig. 6E,F). These results denoted that SlMED18 affects GA biosynthesis and signal transduction.
Figure 5.
The concentration of endogenous gibberellin GA3 (A) and IAA (B) in apical shoots stem of wild type and transgenic lines. We used three wild type simples and three transgenic simples to do biological duplication and the value stand for the mean ± SD of three replicates.
Figure 6.
Genes involved in GAs biosynthesis and signal transduction in SlMED18-RNAi plants. (A–F) Show the expression levels of SlCPS, SlKAO, SlFA20ox1, SlGA3ox2, SlGAST1 and SlGID1 in wild-type and transgenic lines. The relative expression of each gene in the wild-type was set to 1.0 and the Error bar indicates the mean ± SD of three replicates (n = 8). *P < 0.05 and **P < 0.01 according to t-test.
Silencing of SlMED18 alters the expression of auxin transport and response genes
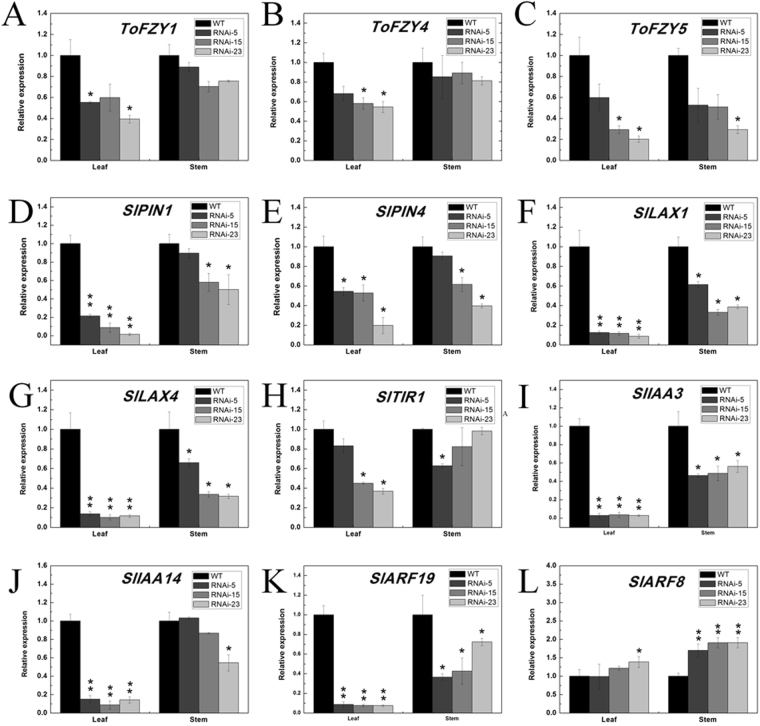
In our study, we compare the expression level of three genes ToFZY1, ToFZY4 and ToFZY5, which contributed to the localized biosynthesis of IAA, between SlMED18-RNAi plants and wild type40. In transgenic plant, ToFZY1, ToFZY4 and ToFZY5 were down-regulated. However, the contents of IAA in the stems of SlMED18-RNAi lines showed no significant change compared to wild type (Fig. 5B). The spatiotemporal localization of auxin acts as an essential regulator of leaves organogenesis and stems growth. The polar auxin transport mediated by PIN and AUX/LAX proteins is a major mechanism that regulates auxin distribution, which controls cellular auxin efflux and influx respectively41. So we further detected the expression of auxin transport and response genes in SlMED18-RNAi plants and wild type. In this study, two PIN genes, PIN1 and PIN4, and two LAX genes, LAX1 and LAX2, were examined in stems and leaves of the transgenic lines and the wild type, respectively. The results demonstrated that these four genes were significantly down-regulated in the stems and leaves of the transgenic lines (Fig. 7D–G). TIR1 is reported to be an auxin receptor that mediates rapid degradation of Aux/IAA proteins and consequently changes the expression of auxin-regulated genes42. In our study, the expression level of SlT1R1 was markedly decreased in leaves of transgenic lines but no significant changes were observed in stems of the transgenic lines compared with the wild type (Fig. 7H). These results may explain the no significant change of IAA content that due to the severe inhibition of auxin transport and the IAA were accumulated at the apices of the stem. We also examined the expression of two genes of Aux/IAA family, SlIAA3 and SlIAA14, which are known to be implicated in modified auxin response. The results displayed that the transcripts of SlIAA3 were remarkably reduced in leaves and stems of transgenic lines and SlIAA14 was significantly down-regulated in leaves but not in stems of transgenic lines (Fig. 7I,J). SlARF19, a transcriptional activator of early auxin response gene, was also significantly reduced in SlMED18-RNAi lines (Fig. 7K).The relative expression level of auxin response gene SlARF8, which may control the free IAA level in a negative feedback fashion43, was up-regulated in the SlMED18-RNAi lines compared with wild type (Fig. 7L). As a result, our study suggested that repression of SlMED18 may affect auxin regulation by regulating the expression of polar auxin transport genes and auxin responsive genes.
Figure 7.
(A–C) The expression levels of three genes ToFZY1, ToFZY4 and ToFZY5, which contributed to the localized biosynthesis of IAA. (D–G) Expression of several key genes of auxin transport and auxin-responsive in SlMED18-RNAi transgenic lines. The relative mRNA level of major polar auxin transport genes SlPINI (D), SlPIN4 (E), SlLAX1 (F) and SlLAX4 (G) in leaf and stem, respectively. (H) Transcript accumulation of TIR1, an auxin receptor. (I–L) The auxin-responsive genes (SlIAA3, SlIAA14, SlARF19 and SlARF8) in leaf and stem tissues were investigated by qRT-PCR. The expression in each sample was used for standardization, and the relative expression of each gene in the wild-type was set to 1.0. Each value represents the mean ± SD of three replicates (n = 8). Asterisks indicate a significant difference (*P < 0.05 and **P < 0.01) between wild type and transgenic lines, according to t-test.
Suppression of SlMED18 results in down-regulated expression of leaf morphogenesis related genes
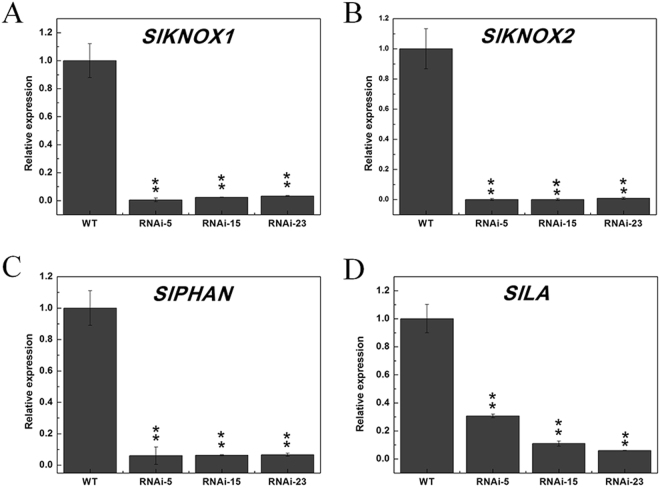
Given that the SlMED18-RNAi transgenic lines had significantly smaller compound leaves with narrower lamina compared with wild type lines. (Fig. 3A and B), we detected the expression of four genes, KNOX1, KNOX2, PHAN and LANCEOLATE (LA), which are associated with leaf morphology and development by qRT-PCR in the leaves of 90-day old wild type and SlMED18-RNAi plants. The results showed that the transcription levels of these genes were sharply decreased in the SlMED18-RNAi lines compared with the wild type (Fig. 8A–D). These results suggested that SlMED18 may affect leaf morphology development through regulating the expression of these key regulators.
Figure 8.
qPCR analysis of key regulators involved in the leaf morphogenesis. The relative expression of SlKNOX1 (A), SlKNOX2 (B), SlPHAN (C) and SlLA (D) showed various changes. The error bar indicates the mean ± SD of three replicates. Asterisks indicate a significant difference (*P < 0.05 and **P < 0.01) as determined by student’s t-test.
Discussion
Mediator complex is a central part of the transcriptional machinery in all eukaryotes. Since the first purification of Mediator complex from yeast, subsequent studies of yeast Mediator subunit mutants expression profiling exposed that some specific sets of genes can be directly regulated by Mediator44. In plants, the research of Mediator is not lagging so far. Over the past few years, a series of reports have emerged revealing the Mediator subunits are essential in regulating diverse processes like embryo pattern formation, developmental timing, flowering and response to multiple different biotic and abiotic stresses15,45,46. However, the information about function of the Mediator subunits on plant developmental processes is lacking and the studies on Mediator subunits among plant kingdom only focus on Arabidopsis thaliana and Oryza sativa. To date, there is little research about the function of Mediator in tomato, which is one of the important model plants.
MED18 is part of the head module of Mediator complex, and some research identified that MED18 plays essential roles in multiple biological functions, including plant responses to microbial infection and regulating flowering and germination by environmental signals in Arabidopsis28,29. Here, phylogenetic analysis among MED18 in tomato, S. cerevisiae (Sc), Human (Hs) and several typical model plants showed that Mediators despite have low sequence homology between S. cerevisiae (Sc), Human (Hs) and plants, but they conserved across plant kingdoms (Fig. 1A,B). In addition, the MED18 was isolated from tomato and expression profile exhibited that SlMED18 was extensively expressed in all of the examined tissues, illustrating the SlMED18 may have effect on different plant physiological processes. Unlike the report in Arabidopsis mutant29, silencing of SlMED18 in tomato caused severe plant developmental defects, including smaller size and slower growth rate of the plant with significantly smaller compound leaves and narrower lamina and smooth margins (Fig. 2B–D and Supplementary Fig. S2A–C). The result suggested that SlMED18 plays important roles in the plant developmental processes.
In SlMED18-RNAi lines, the plant height, width and length of compound leaves and leaf area were measured. The date explicated that the stem and leaf developmental result in a strong reduction in plant growth of whole plant (Figs 2E,F and 3C,D). Moreover, the anatomical characterization of leaf from SlMED18 silenced plants and wild type was performed and we found that the size and number of leaf cell in SlMED18-RNAi lines were drastically decreased (Fig. 4C,D). The genes associated with cell expansion and cell cycle genes, including SlCycA3;1, SlCycB1;1, SlCycD2;1, SlXTH2, SlPRE1, SlPRE2, SlPRE3, SlPRE4 and SlPRE5 (Fig. 4E,F) were remarkable up-regulated in SlMED18-RNAi lines. These results may partially explain the smaller compound leaves in the transgenic lines.
Plant hormones play the crucial roles in regulating plant growth and development as well as plant responses to various biotic and abiotic stress responses. Among the seven different kinds of plant hormones, gibberellin (GAs) and auxin are both key signals in plant developmental and often act synergistically. Some research of dwarf mutants and analysis of their GAs contents made sure that bioactive GAs is associated with the regulation of stem growth in plants47. In tomato, gibberellin-responsive mutants (gib-l, gib-2, and gib-3) were identified by reduced plant height due to shorter internodes and their leaves are smaller, darker green, and structure differently as compared to wild type48. In our study, the endogenous GA3 content of transgenic lines is less than wild type (Fig. 5), demonstrating that the dwarf plant and smaller leaves phenotype of SlMED18-RANi lines may be ascribed to the decreased levels of endogenous GAs. As we all know, the content of endogenous GAs is regulated by the expression of several key genes involved in GAs biosynthesis and signal transduction such as CPS, KAO, SlGA20oxs and SlGA3ox. In previous reports, a number of examples explicated that these genes were involved in alteration of GAs level and lead to a developmental defect plant. Loss-of-function CPS mutants of several plant species, such as Oryza sativa and Arabidopsis thaliana, resulted in severely impaired plant growth49,50. In Arabidopsis thaliana, kao1 kao2 double mutant shows typical non-germinating GA-dwarf phenotypes51. Besides, in tomato, up-regulation of SlGA20oxs, and SlGA3ox can lead to higher levels of bioactive GAs and constitutive co-suppression of the SlGA20ox1 gene cause serious defects in vegetative and reproductive development. In our study, the expression of SlCPS, SlKAO, SlGA20ox1 and SlGA3ox2 were dramatically decreased in leaves and stems of SlMED18-RNAi lines (Fig. 6A–D), demonstrating that repression of SlMED18 lead to severe plant developmental defects by down-regulating the expression of key GA biosynthesis enzyme genes. However, biosynthesis of endogenous bioactive gibberellin (GA) is a complicated process, including the regulated at transcription level, the post-transcriptional level, translation level and post-translational level of key GA biosynthesis enzyme genes. Especially, in eukaryotic, many proteins undergo extensive post-translational modifications such as methylation, acetylation, phosphorylation, glycosylation, and ubiquitination can affect the protein content. For example, ubiquitination has been shown to be involved in the regulation of protein degradation and gene expression. It may explain the reduction in gibberellins contents present in the transgenic line compared with the wild type was not as significant as the expression levels of GA related genes. In addition, the expression levels of a GA response gene SlGAST137 and SlGID1-A were also notably reduced in the leaves of SlMED18-silenced lines but not significantly changed between the wild type and SlMED18-RNAi stems (Fig. 6E,F), probably because of the feedback of GA deficiency.
The plant hormone auxin has a key role in many developmental processes, and it is known that the level of auxin throughout whole plant tissues is tightly controlled the coordination of plant growth and development52. The multidimensional effects of auxin need to coordinate regulation of auxin influx and efflux carriers, guiding the auxin transport in a polar way that together form a polar auxin transport (PAT) network53. Unlike other acknowledged plant hormones, auxin can be actively transported in a directional fashion in order to affect auxin concentrations during phototropic and gravitropic response. Two largest participators of directional transport in the PAT system are the PIN-FORMED (PIN) auxin transporters, which act as auxin efflux carriers, and the AUX1 (AUXIN RESISTANT1) and its close homologues LAX (LIKE AUXIN RESISTANT) that take participate in auxin influx as the influx carrier. The PIN1, PIN4, LAX1, LAX4 genes were dramatically declined (Fig. 7D–G) and several auxin responsive genes were also changed in SlMED18 RNAi lines (Fig. 7H–L). The results collectively suggested that MED18 takes part in the regulation of auxin distribution, possibly through controlling the activity of polar auxin transport genes.
Gibberellin (GA) and auxin are both key signals in plant growth and are often observed to act synergistically54. However, much of our knowledge on GAs and auxin has come from studying them independently. Recently, some studies proposed a new idea that there is crosstalk between GA signalling and auxin transport. There are some reports that the auxin can induce the transcription of several GA biosynthesis genes such as in Arabidopsis, after decapitation the apical source of auxins, the active GA levels in stems are lower than those in stems of intact plants55. In contrast, GA biosynthesis and signalling has a positive impact on proper auxin transport56. In Arabidopsis GA mutants, the GA pathway cross-talk with PIN protein–dependent auxin transport pathway lead to the auxin transport impairment in mutants, which causative for defection of cotyledon differentiation and root gravitropic responses57.
Several genes, including class 1 KNOTTED homeodomain genes (KNOX), PHANTASTICA gene PHAN and CIN-TCP transcription factor LANCEOLATE (LA), have been characterized for their regulatory roles in altering leaf shape and development. PHAN is required for normal meristem function58. The KNOX gene in tomato was involved in organ leaf formation, and the tomato LANCEOLATE (LA) gene was shown to promote leaf differentiation59,60. Silencing SlMED18 in tomato altered the expression of these genes (Fig. 8A–D), suggesting that SlMED18 regulates the leaf development. Interestingly, recently these genes were exhibited to act in part by adjusting gibberellin and auxin levels. KNOX proteins partly linked to GAs pathways and have a positive impact on auxin signalling61. It is reported that expression of the SlGA20ox1 is up-regulated in gain-of-function La mutants, illustrating that LA activity is partly mediated by positive regulation of the GAs response, probably by regulation of endogenous bioactive GAs levels59. In addition, in leaf primordial of harboring the dominant La mutant the auxin signal is very weak, implying that auxin appear to affect the specification of marginal outgrowths, controlled by LA. Thus, narrower lamina of SlMED18-RNAi lines probably not only accounts for the alteration of the key genes for leaf development but also is induced by modulation of GA and auxin signalling. Additionally, we also found the repression of SlMED18 altered inflorescences architecture (Supplementary Fig. A and B), whether this is due to the direct affection on transcriptional regulators or indirect result of hormone signalling pathways, future biochemical analyses will need to identify.
Silencing SlMED18 in tomato caused multiple plant developmental defects, including smaller size and slower growth rate of plant and significantly smaller compound leaves, further we found that SlMED18 affected a set of transcription factors to regulate diverse physiological and cellular processes through impacting intra and extracellular signals. Furthermore, understanding the function of this Mediator subunit in tomato may provide information for entire tomato Mediator complexes.
Materials and Methods
Plant materials and growth conditions
In this article, We planted the tomato (Solanum lycopersicum, ‘Ailsa Craig’ AC++) as wild type that together with transgenic cultures were grown in controlled greenhouse conditions (16-hour-day (25 °C)/8-hour-night (18 °C)cycle, 80% humidity and a 250 μ mol m−2 s−1 light intensity) and managed them routinely. The sample of roots, stems, young leaves, mature leaves, senescent leaves, flowers, sepals and fruits of five periods were collected from tomato for SlMED18 organ-specific expression profiling. Transgenic tomato plants that came from tissue culture in first generation (T0) were used in our experiment because the transgenic plants were sterility. For analysis of gene expression, the fifth leaf from top and the young stem that at the corresponding developmental stage from the 90-day-old plant were collected. Plant samples that we used were frozen in liquid nitrogen immediately then stored at −80 °C.
Phylogenetic Analysis
The cDNA and protein sequence date of tomato mediator of RNA polymerase II transcription subunit 18 was found in NCBI (http://www.ncbi.nlm.nih.gov/) and Sol Genomics Network (SGN, http://solgenomics.net/). MED18 in Solanum lycopersicum (Sl), Arabidopsis thaliana (At), Oryza sativa (Os), Solanum tuberosum (St), Zea mays (Zm), Saccharomyces. cerevisiae (Sc) and Human (Hs) were obtained from the NCBI databases. GenBank accession numbers for multiple sequence alignment and phylogenetic analysis are as follows: SlMED18 (XP_010321804), AtMED18 (NP_565534), OsMED18 (XP_015625319), StMED18 (XP_006364546), ScMED18 (NP_011618) and HsMED18 (NP_001120822). Multiple sequence alignment of MED18 in tomato with other species was conducted by DNAMAN 6.0 programs. Alignments of amino acid sequences of MED18 subunits proteins were made using Clustal X2.162. A phylogenetic tree of Mediator of RNA polymerase II transcription subunit 18 protein sequence in 7 different species was constructed using MEGA7 (http://megasoftware.net/) and the phylogenic analysis was inferred using the Neighbor-Joining method.
Total RNA extraction and quantitative real-time PCR analysis
Using RNAiso Plus (Takara) in accordance with the instructions, we extracted Total RNA from wild type and MED18-RNAi plant tissues. cDNA was synthesized with oligo(dT)20 as primer by RNA that reverse-transcribed using M-MLV reverse transcriptase (Promega). In addition, the synthesized cDNA need to fold dilute three times with nuclease-free water for quantitative real-time PCR analysis. Additionally, the qRT-PCR analysis was conducted with the GoTaq qPCR Master Mix (Promega), 1.0 μL mixture primers, 1.0 μL cDNA, 3.0 μL ddH2O by CFX96™ Real-Time System (Bio-Rad, USA). We performed the NRT (no reverse transcription control) and NTC (no template control) to eliminate the effect by genomic DNA and the environment factor. The organ-specific expression analysis was detected using the SlCAC gene (accession number: SGN-U314153) which showed stable expression across diverse tissues, as internal standard38. The 2−ΔΔCT method was used to performed relative gene expression levels analysis63. In addition, each sample was repeated three times and standard curves were run at the same time. All primers we used were shown in Supplementary Table S1 that designed by Primer premier 6.24 software (http://www.premierbiosoft.com/crm/jsp/com/pbi/crm/clientside/ProductList.jsp).
Expression analysis prediction of SlMED18
SlMED18 expression prediction atlas of tomato tissues were obtained using Tomato lab website (http://tomatolab.cshl.edu/~lippmanlab2/allexp_query.html). In this website, the tomato genome sequence provided form Tomato Genome Consortium and searched by gene ID came from Sol Genomics Network (http://solgenomics.net/)64,65.
SlMED18-RNAi vector construction and plant transformation
To generate MED18-RNAi lines, an RNAi vector was constructed. Primers of SlMED18-RNAi were showed in Supplementary Table S1, tailed with XhoI, XbaI and HindIII, KpnI restriction sites at the 5′end respectively, were used to amplify a 343-bp specific fragment of SlMED18 cDNA. We used cloning vector pHANNIBAL as the original vector, digested the above amplified fragment of SlMED18 products that with HindIII/XbaI and KpnI/XhoI, inserted into the pHANNIBAL plasmid at the HindIII/XbaI restriction site in the sense orientation while at the KpnI/XhoI restriction sited in the antisense orientation. Lastly, a double-stranded RNA expression unit, covered the calf mosaic virus (CaMV) 35 S promoter, was purified and then integrated into a plant binary vector pBIN19 with SacI and XbaI restriction sites. After SlMED18-RNAi Vector constructed, the generated binary plasmids, confirmed by restriction digest analysis and sequencing validation, were transferred into the Agrobacterium tumefactions LBA4404 strain, then the Agrobacterium tumefaciens-mediated transformation was carried out and the SlMED18-RNAi lines were obtained5. The transgenic plants rooted on MS solid medium containing kanamycin for selecting the positive transgenic lines and the primers NPTII-F/-R were used to detect the MED18-RNAi plants (Supplementary Table S1) and the positive transgenic lines were used for further study.
Quantification of phenotypes and statistical analysis
To study the differences between the transgenic plants and wild type, in addition to the height and internode length were measured; the length, width and area of mature leaf (4–5 circles from top) were also calculated. The plant height was measured after cutting shoot rooted on the MS culture medium for 30 days and 30 days after transplanting them into pots, respectively. The other dates were measured form 60-days olds plants planted into pots. Besides, the image-analyzing program Image J (http://rsb.info. nih.gov/ij/) was adapted to measure the length, width and area of compound leaves. Averages and standard errors were calculated from eight different plants.
Anatomical analyses of the leaf
Sectioning of leaf was carried out by hand on mature fresh tissues of 90-day-old plants. Fine cut, with the help of a sharp razor blade and instantly fixed by 70% ethanol/acetic acid/40% formaldehyde (18:1:1, by volume; FAA), dehydrated in gradient ethanol–water series, then fixation, sectioning and dew axing. Cut samples along the middle to prepare the transverse sections of leaves. Finally, we visualized anatomical structure of leaf under a microscope (OLYMPUS IX71) and photographed. The areas of cells and average cell number per area (10000 μm2) were quantified by Image J software (http://rsbweb.nih.gov/ij/).
Quantification of endogenous bioactive gibberellin contents
Collected apical shoots stem and frozen in liquid nitrogen then used for GA3 determination immediately. We used gibberellin (GA3) kit (GA-4-Y Comin Biotechnology Co., Ltd., China) and IAA (IAA-4-C Comin Biotechnology Co., Ltd., China)to extract and purify GA3 and IAA. The HPLC (High Performance Liquid Chromatography) was used to measure the concentration of endogenous bioactive gibberellin (GA3) and IAA in apical shoots stem of wild type and MED18-RNAi lines. Moreover, the experimental operation is strictly related to gibberellin (GA3) kit instruction manual.
Statistical analysis
Data were displayed as mean ± standard deviation. Significant differences between SlMED18-lines and wild type were analyzed by the t-test (**P < 0.01 and *P < 0.05). Considering the biological significance of the differential expression, we used the mean value of the standard deviation (SD) with three biological repeats to represent the measured value.
Electronic supplementary material
Acknowledgements
This work was supported by National Natural Science Foundation of China (nos 30600044, 31572129), and the Natural Science Foundation of Chongqing of China (cstc2015jcyjA80026).
Author Contributions
G.C. and Z.H. designed and managed the research work and improved the manuscript. J.Z., X.Y., J.G., H.L. and J.Z. performed the experiments. Y.W. performed the experiments and wrote the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-21679-1.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Thomas, R. G., Baker, M. J. & Williams, W. M. Vegetative growth and development (1987).
- 2.Hsu CY, Yuceer C. FLOWERING LOCUS T duplication coordinates reproductive and vegetative growth in perennial poplar. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:10756–10761. doi: 10.1073/pnas.1104713108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lemon B, Tjian R. Orchestrated response: a symphony of transcription factors for gene control. Genes & Development. 2000;14:2551–2569. doi: 10.1101/gad.831000. [DOI] [PubMed] [Google Scholar]
- 4.Lee TI, Young RA. Transcription of eukaryotic protein-coding genes. Annual Review of Genetics. 2000;34:77. doi: 10.1146/annurev.genet.34.1.77. [DOI] [PubMed] [Google Scholar]
- 5.Guo X, et al. The MADS-box gene SlMBP11 regulates plant architecture and affects reproductive development in tomato plants. Plant science: an international journal of experimental plant biology. 2017;258:90–101. doi: 10.1016/j.plantsci.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Schumacher K, Schmitt T, Rossberg M, Schmitz G, Theres K. The Lateral suppressor (Ls) Gene of Tomato Encodes a New Member of the VHIID Protein Family. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:290–295. doi: 10.1073/pnas.96.1.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganguli S. The tomato Blind gene encodes a MYB transcription factor that controls the formation of lateral meristems. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:1064–1069. doi: 10.1073/pnas.022516199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kornberg RD. Mediator and the mechanism of transcriptional activation. Trends in Biochemical Sciences. 2005;30:235–239. doi: 10.1016/j.tibs.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Taatjes DJ. The human Mediator complex: a versatile, genome-wide regulator of transcription. Trends in Biochemical Sciences. 2010;35:315–322. doi: 10.1016/j.tibs.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conaway RC, Conaway JW. Origins and Activity of the Mediator Complex. Seminars in Cell & Developmental Biology. 2011;22:729–734. doi: 10.1016/j.semcdb.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rd KR, Flanagan PM, Kornberg RD. A novel mediator between activator proteins and the RNA polymerase II transcription apparatus. Cell. 1990;61:1209–1215. doi: 10.1016/0092-8674(90)90685-8. [DOI] [PubMed] [Google Scholar]
- 12.Chadick JZ, Asturias FJ. Structure of eukaryotic Mediator complexes. Trends in Biochemical Sciences. 2005;30:264–271. doi: 10.1016/j.tibs.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Lariviere L, Seizl M, Cramer P. A structural perspective on Mediator function. Curr Opin Cell Biol. 2012;24:305–313. doi: 10.1016/j.ceb.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Bäckström S, Elfving N, Nilsson R, Wingsle G, Björklund S. Purification of a plant mediator from Arabidopsis thaliana identifies PFT1 as the Med25 subunit. Molecular Cell. 2007;26:717–729. doi: 10.1016/j.molcel.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Gillmor CS, et al. The MED12-MED13 module of mediator regulates the timing of embryo patterning in Arabidopsis. Development. 2010;137:113–122. doi: 10.1242/dev.043174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imura Y, et al. Cryptic precocious/med12 is a novel flowering regulator with multiple target steps in Arabidopsis. Plant & Cell Physiology. 2012;53:287. doi: 10.1093/pcp/pcs002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cerdán PD, Chory J. Regulation of flowering time by light quality. Nature. 2003;423:881. doi: 10.1038/nature01636. [DOI] [PubMed] [Google Scholar]
- 18.Kidd BN, et al. The mediator complex subunit PFT1 is a key regulator of jasmonate-dependent defense in Arabidopsis. Plant Cell. 2009;21:2237–2252. doi: 10.1105/tpc.109.066910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu R, Li Y. Control of final organ size by Mediator complex subunit 25 in Arabidopsis thaliana. Development. 2011;138:4545–4554. doi: 10.1242/dev.071423. [DOI] [PubMed] [Google Scholar]
- 20.Chen R, et al. The Arabidopsis mediator subunit MED25 differentially regulates jasmonate and abscisic acid signaling through interacting with the MYC2 and ABI5 transcription factors. The Plant Cell. 2012;24:2898–2916. doi: 10.1105/tpc.112.098277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iñigo S, Giraldez AN, Chory J, Cerdán PD. Proteasome-mediated turnover of Arabidopsis MED25 is coupled to the activation of FLOWERING LOCUS T transcription. Plant Physiology. 2012;160:1662–1673. doi: 10.1104/pp.112.205500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez D, Bowen AJ, Carroll TS, Conlan RS. The transcription corepressor LEUNIG interacts with the histone deacetylase HDA19 and mediator components MED14 (SWP) and CDK8 (HEN3) to repress transcription. Molecular & Cellular Biology. 2007;27:5306. doi: 10.1128/MCB.01912-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim YJ, et al. The role of Mediator in small and long noncoding RNA production in Arabidopsis thaliana. The EMBO journal. 2011;30:814–822. doi: 10.1038/emboj.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wathugala DL, et al. The Mediator subunit SFR6/MED16 controls defence gene expression mediated by salicylic acid and jasmonate responsive pathways. New Phytologist. 2012;195:217–230. doi: 10.1111/j.1469-8137.2012.04138.x. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Wang C, Zhang Y, Sun Y, Mou Z. The Arabidopsis mediator complex subunit16 positively regulates salicylate-mediated systemic acquired resistance and jasmonate/ethylene-induced defense pathways. Plant Cell. 2012;24:4294–4309. doi: 10.1105/tpc.112.103317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canet JV, Dobón A, Tornero P. Non-Recognition-of-BTH4, an Arabidopsis Mediator Subunit Homolog, Is Necessary for Development and Response to Salicylic Acid. Plant Cell. 2012;24:4220–4235. doi: 10.1105/tpc.112.103028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathur S, Vyas S, Kapoor S, Tyagi AK. The Mediator complex in plants: structure, phylogeny, and expression profiling of representative genes in a dicot (Arabidopsis) and a monocot (rice) during reproduction and abiotic stress. Plant Physiology. 2011;157:1609–1627. doi: 10.1104/pp.111.188300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng Z, Guan H, Leal F, Grey PH, Oppenheimer DG. Mediator subunit18 controls flowering time and floral organ identity in Arabidopsis. PloS one. 2013;8:e53924. doi: 10.1371/journal.pone.0053924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lai Z, et al. MED18 interaction with distinct transcription factors regulates multiple plant functions. Nature Communications. 2014;5:3064. doi: 10.1038/ncomms4064. [DOI] [PubMed] [Google Scholar]
- 30.Liao CJ, Lai Z, Lee S, Yun DJ, Mengiste T. Arabidopsis HOOKLESS1 Regulates Responses to Pathogens and Abscisic Acid through Interaction with MED18 and Acetylation of WRKY33 and ABI5 Chromatin. Plant Cell. 2016;28:1662. doi: 10.1105/tpc.16.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang T, et al. Genome-Wide Analysis of the Cyclin Gene Family in Tomato. International Journal of Molecular Sciences. 2013;15:120. doi: 10.3390/ijms15010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saladié M, Rose JKC, Cosgrove DJ, Catalá C. Characterization of a new xyloglucan endotransglucosylase/hydrolase (XTH) from ripening tomato fruit and implications for the diverse modes of enzymic action. Plant Journal for Cell & Molecular Biology. 2010;47:282–295. doi: 10.1111/j.1365-313X.2006.02784.x. [DOI] [PubMed] [Google Scholar]
- 33.Zhu Z, et al. Overexpression of SlPRE2, an atypical bHLH transcription factor, affects plant morphology and fruit pigment accumulation in tomato. Sci Rep. 2017;7:5786. doi: 10.1038/s41598-017-04092-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swain SM, Olszewski NE. Genetic Analysis of Gibberellin Signal Transduction. Plant Physiology. 1996;112:11. doi: 10.1104/pp.112.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Schie CC, et al. Geranyl diphosphate synthase is required for biosynthesis of gibberellins. Plant Journal for Cell & Molecular Biology. 2007;52:752–762. doi: 10.1111/j.1365-313X.2007.03273.x. [DOI] [PubMed] [Google Scholar]
- 36.Yamaguchi S. Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol. 2008;59:225–251. doi: 10.1146/annurev.arplant.59.032607.092804. [DOI] [PubMed] [Google Scholar]
- 37.Shi L, Olszewski NE. Gibberellin and abscisic acid regulate GAST1 expression at the level of transcription. Plant molecular biology. 1998;38:1053–1060. doi: 10.1023/A:1006007315718. [DOI] [PubMed] [Google Scholar]
- 38.Exposito-Rodriguez M, Borges AA, Borges-Perez A, Perez JA. Selection of internal control genes for quantitative real-time RT-PCR studies during tomato development process. BMC Plant Biol. 2008;8:131. doi: 10.1186/1471-2229-8-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cui B, et al. Overexpression of SlUPA-like induces cell enlargement, aberrant development and low stress tolerance through phytohormonal pathway in tomato. Scientific Reports. 2016;6:23818. doi: 10.1038/srep23818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Exposito-Rodriguez M, Borges AA, Borges-Perez A, Perez JA. Gene structure and spatiotemporal expression profile of tomato genes encoding YUCCA-like flavin monooxygenases: the ToFZY gene family. Plant physiology and biochemistry: PPB. 2011;49:782–791. doi: 10.1016/j.plaphy.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 41.Martinez CC, Koenig D, Chitwood DH, Sinha NR. A sister of PIN1 gene in tomato (Solanum lycopersicum) defines leaf and flower organ initiation patterns by maintaining epidermal auxin flux. Developmental biology. 2016;419:85–98. doi: 10.1016/j.ydbio.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 42.Parry G, Estelle M. Auxin receptors: a new role for F-box proteins. Current Opinion in Cell Biology. 2006;18:152–156. doi: 10.1016/j.ceb.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Tian CE, et al. Disruption and overexpression of auxin response factor 8 gene of Arabidopsis affect hypocotyl elongation and root growth habit, indicating its possible involvement in auxin homeostasis in light condition. Plant Journal. 2004;40:333–343. doi: 10.1111/j.1365-313X.2004.02220.x. [DOI] [PubMed] [Google Scholar]
- 44.Peppel JVD, et al. Mediator Expression Profiling Epistasis Reveals a Signal Transduction Pathway with Antagonistic Submodules and Highly Specific Downstream Targets. Molecular Cell. 2005;19:511–522. doi: 10.1016/j.molcel.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 45.Dhawan R, et al. HISTONE MONOUBIQUITINATION1 interacts with a subunit of the mediator complex and regulates defense against necrotrophic fungal pathogens in Arabidopsis. Plant Cell. 2009;21:1000–1019. doi: 10.1105/tpc.108.062364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elfving N, et al. The Arabidopsis thaliana Med25 mediator subunit integrates environmental cues to control plant development. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:8245–8250. doi: 10.1073/pnas.1002981108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lange T. Molecular biology of gibberellin synthesis. Planta. 1998;204:409–419. doi: 10.1007/s004250050274. [DOI] [PubMed] [Google Scholar]
- 48.Sponsel, V. M. & Hedden, P. Gibberellin Biosynthesis and Inactivation. (Springer Netherlands, 2010).
- 49.Prisic S, Xu M, Wilderman PR, Peters RJ. Rice contains two disparate ent-copalyl diphosphate synthases with distinct metabolic functions. Plant Physiology. 2004;136:4228–4236. doi: 10.1104/pp.104.050567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun T, Kamiya Y. The Arabidopsis GA1 locus encodes the cyclase ent-kaurene synthetase A of gibberellin biosynthesis. The Plant Cell. 1994;6:1509–1518. doi: 10.1105/tpc.6.10.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Regnault T, Heintz D, Lange T, Achard P. The gibberellin biosynthetic genes AtKAO1 and AtKAO2 have overlapping roles throughout Arabidopsis development. Plant Journal for Cell & Molecular Biology. 2014;80:462–474. doi: 10.1111/tpj.12648. [DOI] [PubMed] [Google Scholar]
- 52.Friml J. Auxin transport — shaping the plant. Current Opinion in Plant Biology. 2003;6:7–12. doi: 10.1016/S1369526602000031. [DOI] [PubMed] [Google Scholar]
- 53.Zazimalova E, Murphy AS, Yang H, Hoyerova K, Hosek P. Auxin transporters–why so many? Cold Spring Harbor perspectives in biology. 2010;2:a001552. doi: 10.1101/cshperspect.a001552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Swarup R, Parry G, Graham N, Allen T, Bennett M. Auxin cross-talk: integration of signalling pathways to control plant development. Plant Molecular Biology. 2002;49:411. doi: 10.1023/A:1015250929138. [DOI] [PubMed] [Google Scholar]
- 55.Friml J, Palme K. Polar auxin transport – old questions and new concepts? Plant Molecular Biology. 2002;49:273. doi: 10.1023/A:1015248926412. [DOI] [PubMed] [Google Scholar]
- 56.Li G, Zhu C, Gan L, Ng D, Xia K. GA 3 enhances root responsiveness to exogenous IAA by modulating auxin transport and signalling in Arabidopsis. Plant Cell Reports. 2015;34:483–494. doi: 10.1007/s00299-014-1728-y. [DOI] [PubMed] [Google Scholar]
- 57.Willige BC, Isono E, Richter R, Zourelidou M, Schwechheimer C. Gibberellin regulates PIN-FORMED abundance and is required for auxin transport-dependent growth and development in Arabidopsis thaliana. Plant Cell. 2011;23:2184–2195. doi: 10.1105/tpc.111.086355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koltai H, Bird MK. Epistatic repression of PHANTASTICA and class 1 KNOTTED genes is uncoupled in tomato. Plant Journal. 2000;22:455–459. doi: 10.1046/j.1365-313X.2000.00754.x. [DOI] [PubMed] [Google Scholar]
- 59.Yanai O, Shani E, Russ D, Ori N. Gibberellin partly mediates LANCEOLATE activity in tomato. Plant Journal. 2011;68:571–582. doi: 10.1111/j.1365-313X.2011.04716.x. [DOI] [PubMed] [Google Scholar]
- 60.Bengera H, Ori N. Auxin and LANCEOLATE affect leaf shape in tomato via different developmental processes. Plant Signaling & Behavior. 2012;7:1255–1257. doi: 10.4161/psb.21550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsuda K, Hake S. Diverse functions of KNOX transcription factors in the diploid body plan of plants. Current Opinion in Plant Biology. 2015;27:91–96. doi: 10.1016/j.pbi.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 62.Kroes, H. et al. Clustal W and Clustal X version 2.0. Bioinformatics. Clustal W and Clustal X version 2.0. Bioinformatics23, 2947–2948 (2007). [DOI] [PubMed]
- 63.Kenneth, J. & Livak, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 - ct method. Method (2001). [DOI] [PubMed]
- 64.Consortium TG. The tomato genome sequence provides insights into fleshy fruit evolution. Nature. 2012;485:635. doi: 10.1038/nature11119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Becker A, Theißen G. The major clades of MADS-box genes and their role in the development and evolution of flowering plants. Molecular Phylogenetics & Evolution. 2003;29:464–489. doi: 10.1016/S1055-7903(03)00207-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.