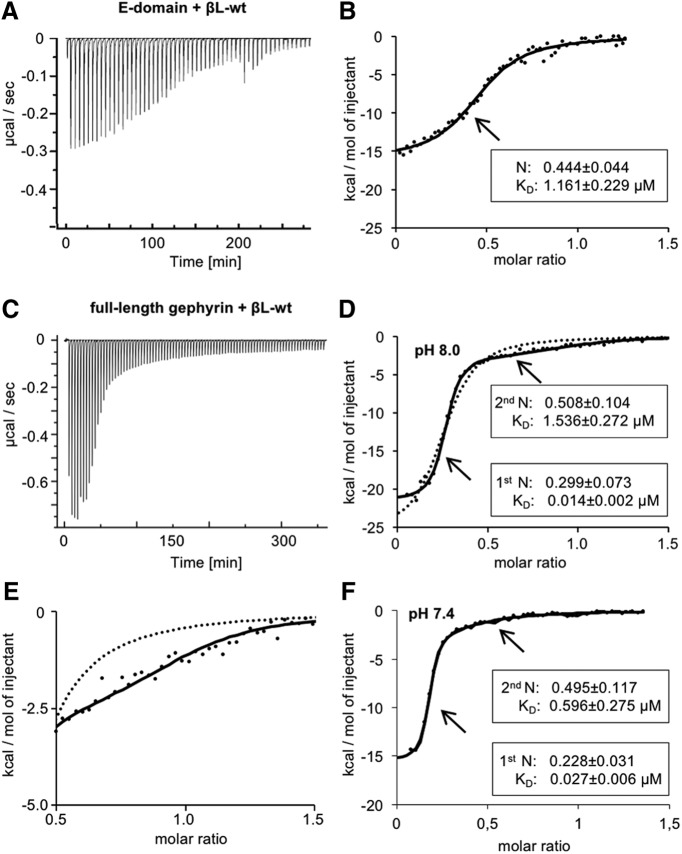
Figure 1.
Binding properties of GlyR β-loop to full-length gephyrin or the isolated E-domain. A, Representative ITC titration profile of βL-wt (378–426; 281 µM) into GephE (31 µM) at pH 8.0. The recorded peaks were corrected by baseline-corrected injection heats. B, Binding isotherms (dots) of integrated binding heats were fitted to a one-site model (black line). The average dissociation constant (KD) and binding stoichiometry with GephE (N) of five independent experiments are given. C, Representative ITC titration profile of βL-wt (327 µM) into gephyrin (29 µM). D, Binding isotherm (dots) of integrated binding heats were fitted to a two-site model (black line) or a one-site model (dotted line). An individual measurement of βL-wt binding to gephyrin is shown alongside with averaged thermodynamic parameters of both sites (binding stoichiometry N and dissociation constant KD). Binding enthalpies (ΔH in kcal/mol) for βL-wt high and low affinity were compared using an unpaired two-tailed t test: p = 0.0005 βL-wt high-affinity site n = 3 versus βL-wt low-affinity site n = 3. E, Magnification of the graph represented in D, showing the fitted curves of the binding isotherm of βL-wt and gephyrin (dots) derived from the two-site (black line) or the one-site (dotted line) binding model. F, ITC data showing the bimodal binding between βL-wt and gephyrin at pH 7.4. Binding isotherms of βL-wt (378–426; 248 µM) into gephyrin (28.6 µM) at pH 7.4. Binding isotherms (dots) of integrated binding heats were fitted to a two-site model (black line). An individual measurement of βL-wt binding to gephyrin is shown alongside with averaged thermodynamic parameters of both sites (binding stoichiometry N and dissociation constant KD). Binding affinities and enthalpies for βL-wt high- and low-affinity binding sites at pH 8.0 and 7.4 were compared using an unpaired two-tailed t test: p = 0.2143 KD βL-wt high-affinity sites n = 3; p = 0.0958 KD βL-wt low-affinity sites n = 3; p = 0.1889 ΔH βL-wt high-affinity sites n = 3; p = 0.0849 ΔH βL-wt low-affinity sites n = 3.