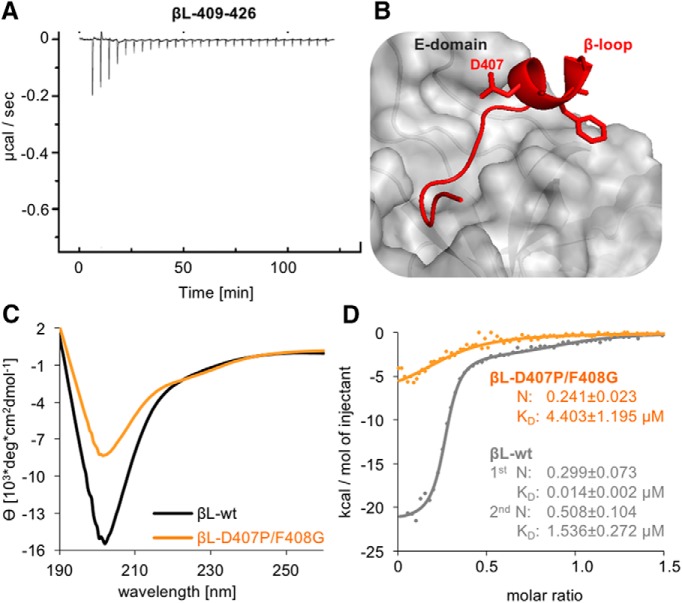
Figure 4.
Impact of the GlyR β-loop conformation on gephyrin binding. A, ITC titration profile of βL409–426 (445 µM) into gephyrin (25 µM). B, The GlyR β-loop in association with GephE adopts a short 310-helix formed by residues 406–410 (Kim et al., 2006). Two residues, Asp407 and Phe408, were mutated to proline and glycine (βL-D407P/F408G) to block the formation of the 310-helix. C, βL-wt and βL-D407P/F408G (both 0.21 mg/ml) folding was compared by CD spectroscopy. The mean residue ellipticity (θ) was plotted against the respective wavelength. D, Comparison of the binding isotherms of representative measurements using 327 µM βL-wt peptide (gray dots, same data as in Fig. 1D,E) and 315 µM βL-D407P/F408G (orange dots) with 29 or 32 µM gephyrin, respectively. Curve calculation was performed based on a two-site model for βL-wt (gray line) and a one-site model for βL-D407P/F408G (orange line). Data were compared using an unpaired two-tailed t test: p = 0.0267 KD of βL-wt high-affinity site n = 3 versus βL-D407P/F408G n = 4.