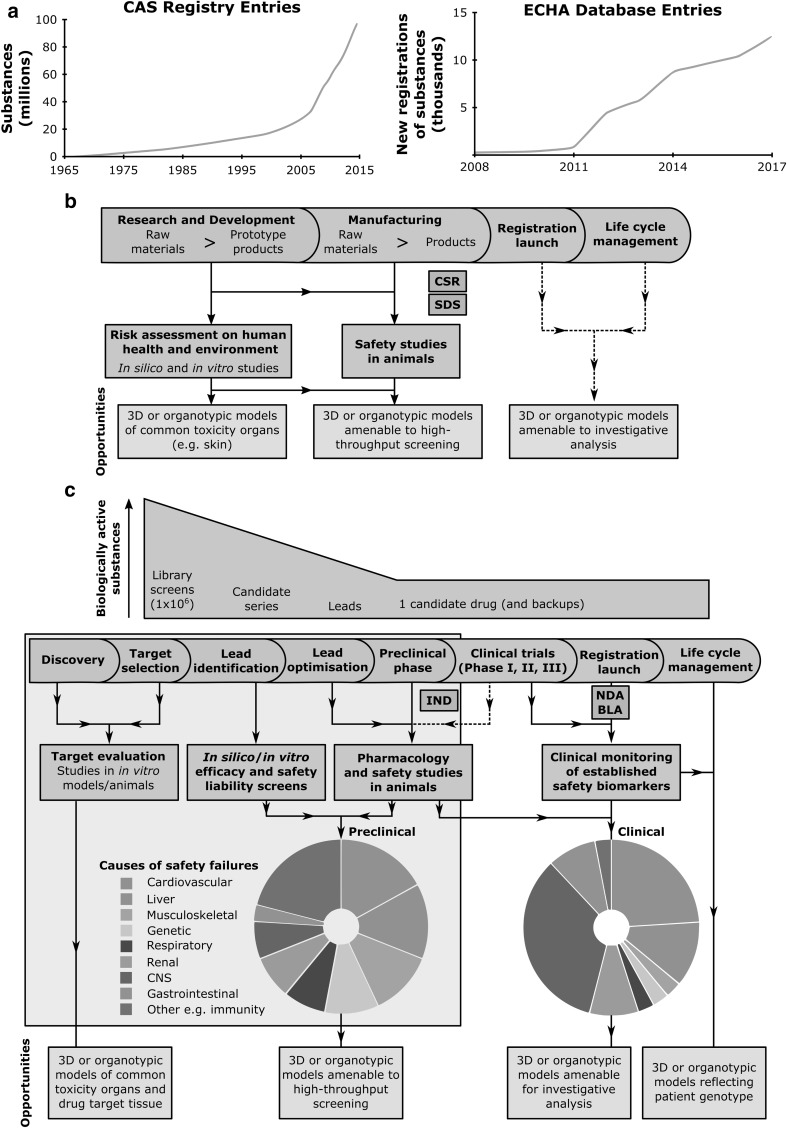
Fig. 1.
Safety assessments during the development of chemicals or drugs and opportunities for the application of innovative in vitro models. a Number of newly synthesized organic and inorganic chemical substances recorded in the CAS Registry (Kemsley 2015) and number of substances registered at the European Chemicals Agency (ECHA). b Schematics of the development process for chemical substances including safety assessments. Violet squares show interactions with regulatory authorities with respect to chemical safety reports (CSR) and the provision of safety data sheets (SDS). Opportunities for the application of 3D or organotypic in vitro models are indicated. c Schematics of the drug development pipeline from the identification of safety liabilities during discovery, screening, and early development to risk/benefit assessments during clinical trials and product life cycle management. Violet squares show important interactions with regulatory authorities, e.g., Investigational New Drug (IND) applications and New Drug or Biologic License Applications (NDA/BLA). Indicated are the major organ systems involved in pre-clinical and clinical safety failures (Cook et al. 2014) as well as opportunities for the application of 3D or organotypic in vitro models. (Color figure online)