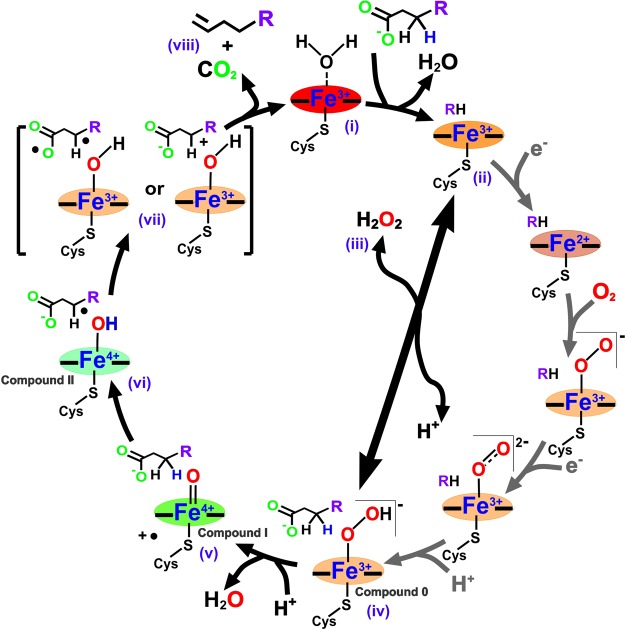
Figure 2. The cytochrome P450 catalytic cycle, incorporating steps specific to peroxygenase activity and the formation of terminal alkenes.
(i) The P450 is in a low-spin ferric resting form with its heme iron axially co-ordinated by cysteine thiolate and a H2O molecule. (ii) Substrate (fatty acid) binding displaces the axial water and converts the heme iron from low-spin to a high-spin form. (iii) Peroxygenase P450s use the peroxide shunt pathway that bypasses the need for external electrons, and interaction with H2O2 facilitates direct conversion of the substrate-bound, high-spin P450 to the ferric-hydroperoxo species (compound 0) (iv). Subsequent protonation and dehydration steps yield the reactive ferryl (FeIV)-oxo porphyrin radical cation (compound I) (v), as observed in rapid mixing studies of OleT with perdeuterated arachidic acid substrate [40]. (vi) Compound I abstracts a hydrogen atom from the Cβ position, resulting in formation of a substrate radical and the ferryl (FeIV)-hydroxo species (compound II) [41]. (vii) Decarboxylation of the fatty acid occurs through subsequent one-electron oxidation of the substrate by compound II to generate a substrate diradical species or a carbocation. (viii) The reactive species can readily eliminate CO2, with formation of the Cα–Cβ double bond and generation of a terminal alkene. The simultaneous recruitment of a proton to the heme restores the resting, distal water-ligated state of the P450. There is competition between the ‘radical rebound’ reaction in which (a) compound II transfers a hydroxyl radical to the substrate radical to form a hydroxylated fatty acid and the alkene-producing reaction in which (b) compound II abstracts a substrate electron to initiate fatty acid decarboxylation. The decarboxylase reaction dominates in OleT for longer chain substrates, although significant levels of hydroxylation are seen with short chain fatty acids. The P450 heme (illustrated by the iron in the relevant oxidation state and equatorial/axial bonds) is shaded to approximate the color of the relevant heme intermediate species.