Summary
Objective
Previous work has shown that high body mass index (BMI) is associated with low grey matter volume. However, evidence on the relationship between waist circumference (WC) and brain volume is relatively scarce. Moreover, the influence of mild obesity (as indexed by WC and BMI) on brain volume remains unclear. This study explored the relationships between WC and BMI and grey matter volume in a large sample of Japanese adults.
Methods
The participants were 792 community‐dwelling adults (523 men and 269 women). Brain magnetic resonance images were collected, and the correlation between WC or BMI and global grey matter volume were analysed. The relationships between WC or BMI and regional grey matter volume were also investigated using voxel‐based morphometry.
Results
Global grey matter volume was not correlated with WC or BMI. Voxel‐based morphometry analysis revealed significant negative correlations between both WC and BMI and regional grey matter volume. The areas correlated with each index were more widespread in men than in women. In women, the total area of the regions significantly correlated with WC was slightly greater than that of the regions significantly correlated with BMI.
Conclusions
Results show that both WC and BMI were inversely related to regional grey matter volume, even in Japanese adults with somewhat mild obesity. Especially in populations with less obesity, such as the female participants in current study, WC may be more sensitive than BMI as a marker of grey matter volume differences associated with obesity.
Keywords: Body fat distribution, body mass index, brain, waist circumference
Introduction
Obesity is a risk factor for neurodegenerative diseases, such as Alzheimer's disease 1, 2, 3, as well as for hypertension 4, 5, coronary heart disease 6 and metabolic diseases 7, 8. Body mass index (BMI), which has conventionally been used to measure excess body fat, is associated with cerebral atrophy of the temporal region, as evaluated by visual ratings of computed tomography scans 9. Several magnetic resonance imaging studies of healthy participants have revealed specific brain regions in which reduced volume is associated with BMI 10, 11, 12, 13. These studies indicate that alterations in specific brain structures associated with obesity may precede clinically significant neurological changes in the brain.
Although widely used, the appropriateness of BMI as a universal indicator of body fatness for all populations has been questioned 7, 14. BMI does not accurately measure fat content, nor does it reflect the proportions of muscle and fat, or account for sex and racial differences in fat content and distribution of intra‐abdominal (visceral) and subcutaneous fat 7, 15. The risk of high mortality with normal‐weight central obesity may be overlooked when only the BMI is used 14. Among the US population, the mortality of metabolically unhealthy people with a normal BMI is higher than that of metabolically healthy people classified as obese by the BMI 7. In Asia in particular, a great concern is that World Health Organization (WHO)‐defined BMI cut‐offs 16 may underestimate the risk from obesity because Asians tend to have increased body fat at normal BMI values 7, 17. Shiwaku et al. reported that Japanese with BMIs in the range of 23.0–24.9 are at increased risk for obesity‐associated disorders, even though these values are classified as normal according to WHO criteria 17.
Recently, waist circumference (WC), which estimates abdominal fat more directly than does BMI, has been argued to be a better indicator than BMI because WC is more closely correlated with the secondary adverse effects of obesity 18, 19, 20. Several previous studies have investigated the relationship between WC and brain structure 10, 21, 22. Kurth et al. demonstrated negative correlations between WC and grey matter volume and between BMI and grey matter volume in several brain regions, including the hypothalamus; the prefrontal, anterior temporal and inferior parietal cortices; and the cerebellum, with women showing more widespread correlations for WC than for BMI 10. Participants in previous studies were Caucasian, although this was not explicitly stated in the study by Debette et al. 21. Only the study by Kurth et al. 10 showed the actual number of participants with obesity (10% [11/115] with a BMI ≥ 30). Indeed, the incidence of obesity, as defined by the WHO criteria of BMI of 30 or greater, is estimated to be 10–20% in Europe and the USA, in contrast to 2–3% in Japan 23. Therefore, evidence on the relationship between WC and brain structure in populations with less obesity, such as the Japanese, remains sparse.
This study investigated correlations between brain volumes and obesity in a large Japanese sample, using both WC and BMI. These correlations for the global grey matter volume and for specific grey matter regions were calculated using voxel‐based morphometry (VBM). Based on the previous studies mentioned previously, the hypothesis was examined that obesity‐related differences in grey matter volume will be seen among individuals of a population with less obesity and that WC may have advantages over BMI in this population. This study also focused on sex‐associated differences because sex is a key demographic factor that influences eating behaviour and body‐weight regulation 24.
Methods
Participants
The participants were volunteers who had undergone private health screening at the University of Tokyo Hospital between 2008 and 2009. The body weight, height and score on the Mini‐Mental State Examination (MMSE) were measured as part of the health screening visit. WC was measured at the umbilicus level, according to the Japanese definition 25. The BMI was calculated as weight divided by height squared (kg m−2). In addition, blood pressure and blood samples, including blood sugar as well as serum levels of lipids, insulin and adiponectin, were evaluated. Patients were defined as having metabolic syndrome when they met the criteria for Japanese metabolic syndrome 25 – central obesity (a WC of 85 cm or more for men and 90 cm or more for women) and any two of the following three risk factors: serum triglycerides ≥150 mg dL−1, serum high‐density lipoprotein cholesterol <40 mg dL−1 or both; systolic blood pressure ≥130 mmHg, diastolic blood pressure ≥85 mmHg or both; and fasting blood glucose levels ≥110 mg dL−1. The homeostasis model assessment of insulin resistance (HOMA‐IR) index was given as fasting insulin (μIU mL−1) × fasting glucose (mmol L−1), and the level of insulin resistance was defined as HOMA‐IR > 2.5 26. Although the existence of metabolic syndrome and that of insulin resistance was not considered exclusion criteria here, to include individuals with overweight and obesity in the study, these criteria were used later to divide participants into subgroups to check the potential influence of these parameters on global grey matter volume.
Participants who had a history of neuropsychiatric disorder or central nervous system disease were excluded. Two trained neuroradiologists reviewed all scans (including T2‐weighted and fluid‐attenuated inversion recovery images), and participants who had old infarcts, haemorrhages or aneurysms were excluded. The inclusion criterion for Fazekas et al. visual scale score to assess white matter on magnetic resonance imaging (range, 0 to 3) 27 was restricted to between 0 (absence) and 2 (smooth ‘halo’). The institutional ethics committee approved the study. This study complies with the principles of the Declaration of Helsinki. Written informed consent was obtained from each participant after providing a complete explanation of the study. Furthermore, to protect subject confidentiality, patient information was stripped from all data.
Image acquisition
Magnetic resonance imaging data were obtained on two 3T Signa HDx scanners (GE Medical Systems, Milwaukee, Wisconsin, USA) of the exact same model with an 8‐channel brain phased‐array coil. For the VBM analysis, T1‐weighted images were acquired in 124 slices by using three‐dimensional spoiled‐gradient recalled acquisition in the steady state (repetition time, 6.4 ms; echo time, 2.0 ms; flip angle, 151; field of view, 250 mm; slice thickness, 1 mm with no gap; acquisition matrix, 256 × 256; number of excitations, 0.5). The voxel dimensions were 0.977 × 0.977 × 1.0 mm.
Image analysis
All three‐dimensional spoiled‐gradient images were processed and examined by using the Statistical Parametric Mapping version 8 (spm8) software (Wellcome Department of Imaging Neuroscience, London, UK; http://www.fil.ion.ucl.ac.uk/spm), in which VBM implemented in the VBM8 toolbox (http://dbm.neuro.uni‐jena.de/vbm.html) with default parameters in matlab 7.7.0.471 (The MathWorks, Natick, MA, USA) running on a Windows computer.
A ‘nonlinear only’ modulation was performed on all images during spatial normalization to express the values in the resultant images as volumes corrected for brain size. The resultant modulated images were smoothed by using a Gaussian kernel of 8 mm (full width at half maximum). In addition, spm8 default modulation was performed to calculate the total intracranial volume (TIV) as the sum of grey matter, white matter and cerebrospinal fluid volumes. When analysing global grey matter volume, the grey matter fraction (GMF) was defined as the proportion of the TIV occupied by the grey matter volume, to normalize the head size of each subject.
Statistical analysis
Pearson product moment correlations between GMF and WC, BMI, age, MMSE, as well as TIV were calculated separately for each sex to investigate the relationships between global grey matter volume and the other variables. The significance level was set at P < 0.05.
Voxel‐wise analyses were performed to investigate the correlation between WC or BMI and the regional grey matter volume. Multiple regression was performed in spm8 separately for each sex. The WC or BMI was treated as a covariate‐of‐interest. As nuisance variables, individual values for age and MMSE were included in the analysis for each sex. Two linear contrasts (1, −1) were made for positive and negative correlations, respectively. The significance level was set at the family‐wise error‐corrected P value of less than 0.05.
Results
Characteristics of the study population
Data from 792 participants (523 men and 269 women) were included in the analyses (Table 1). There were no sex differences in age or MMSE. The WC and BMI were significantly different between men and women, with both being greater in men than in women (P < 0.0001 for both; Wilcoxon rank‐sum test). The TIV was also larger in men than in women (P < 0.0001; Student's t‐test). In contrast, the GMF was larger in women than in men (P < 0.0001; Wilcoxon rank‐sum test). There was no sex difference in the prevalence of people who qualified as obese with a BMI ≥ 30. However, the prevalence of participants with each of the following conditions was significantly lower in women than in men: those who (i) qualified as overweight with a BMI between 25 and 30, (ii) met the criteria for metabolic syndrome, (iii) met the criteria for insulin resistance or (iv) had a treatment history of lifestyle‐related diseases (all Ps < 0.01; χ 2 test).
Table 1.
Characteristics of study participants
| Men (N = 523) | Women (N = 269) | P | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | ||
| Age | 55.3 | 9.7 | 23–84 | 55.2 | 9.9 | 24–81 | n.s. |
| MMSE | 29.1 | 1.1 | 24–30 | 29.2 | 1 | 24–30 | n.s. |
| WC (cm) | 88.5 | 8.1 | 64–127 | 81.2 | 9.8 | 58–113 | <0.0001 |
| BMI (kg m−2) | 24.7 | 3.1 | 15.8–41.2 | 22 | 3.3 | 14.4–34.3 | <0.0001 |
| Adiponectin (μg mL−1) | 6.9 | 3.7 | 0.93–26.8 (N = 499) | 11.3 | 5.7 | 0.83–37.9 (N = 258) | <0.0001 |
| TIV (mL) | 1477 | 95 | 1,233–1,784 | 1305 | 92 | 998–1,578 | <0.0001 |
| GMF | 0.43 | 0.021 | 0.36–0.49 | 0.447 | 0.018 | 0.38–0.49 | <0.0001 |
| N | % | N | % | P | |||
| Prevalence of obesity | |||||||
| 25 ≤ BMI < 30 | 203 | 39 | 33 | 12 | <0.01 | ||
| 30 ≤ BMI | 21 | 4 | 8 | 3 | n.s. | ||
| Metabolic syndrome | 107 | 20 | 6 | 2 | <0.01 | ||
| Insulin resistance | 95 | 18 | 4 | 1 | <0.01 | ||
| Treatment of lifestyle disease | 39 | 7 | 5 | 2 | <0.01 | ||
The sample sizes for adiponectin were smaller due to missing values.
BMI, body mass index; GMF, grey matter fraction; MMSE, Mini‐Mental State Examination; n.s., non‐significant; SD, standard deviation; TIV, total intracranial volume; WC, waist circumference.
Relationships of global grey matter volume to other variables
Table 2 shows Pearson's correlation coefficients between variables for each sex. Neither WC nor BMI was significantly correlated with GMF. This was also the case when the partial correlation coefficients between WC or BMI and GMF adjusted for age, MMSE and TIV were analysed. Age‐related increases in WC and BMI were seen only in female participants. The partial correlation coefficient adjusted for MMSE and TIV was also significant between WC and age (r = 0.28, P < 0.01) and between BMI and age (r = 0.16, P < 0.01) in female participants. GMF and TIV were negatively correlated with adiponectin in male participants only, although the correlation became non‐significant when adjusted for age (r = −0.08, P = 0.09; r = −0.04, P = 0.34). The participants were further divided into subgroups with or without metabolic syndrome or insulin resistance to check whether GMF was related to WC or BMI when analysed separately for each subgroup. However, none of the correlations reached statistical significance.
Table 2.
Pearson product moment correlations between variables for each sex
| WC | BMI | Age | MMSE | TIV | Adiponectin | |
|---|---|---|---|---|---|---|
| Women | ||||||
| BMI | 0.87** | |||||
| Age | 0.28** | 0.16** | ||||
| MMSE | −0.03 | −0.05 | −0.31** | |||
| TIV | 0.02 | −0.01 | −0.12* | 0.17** | ||
| Adiponectin | −0.26** | −0.27** | 0.16* | −0.10 | −0.03 | |
| GMF | −0.07 | 0.01 | −0.50** | 0.17** | −0.16** | −0.10 |
| Men | ||||||
| BMI | 0.87** | |||||
| Age | 0.05 | −0.07 | ||||
| MMSE | −0.07 | −0.06 | −0.26** | |||
| TIV | 0.04 | 0.07 | −0.22** | 0.13** | ||
| Adiponectin | −0.24** | −0.28** | 0.27** | −0.08 | −0.10* | |
| GMF | −0.05 | 0.01 | −0.61** | 0.21** | −0.05 | −0.22** |
P < 0.01,
P < 0.05.
BMI, body mass index; GMF, grey matter fraction; MMSE, Mini‐Mental State Examination; TIV, total intracranial volume; WC, waist circumference.
Voxel‐wise analysis with voxel‐based morphometry
In male participants, widespread regions in which grey matter was negatively correlated with WC or BMI were observed: the temporal lobes, thalamus, rectal gyrus, frontal gyrus, precentral gyrus, lingual gyrus, precuneus, posterior cingulate, postcentral gyrus, inferior parietal lobule, cingulate gyrus, insula and superior temporal gyrus (Table 3 and Figure 1, blue). For female participants, significant WC‐related or BMI‐related decreases in regional grey matter volume were also observed, although the areas were much smaller than in male participants (Table 4 and Figure 1, red). The sum of the significant cluster sizes (number of voxels) for WC was close to that for BMI, although the former was slightly larger than the latter in female participants (WC = 2,358 and BMI = 2,136) and smaller than the latter in male participants (WC = 47,383 and BMI = 53,544).
Table 3.
MNI coordinates and grey matter regions negatively correlated with WC or BMI in male participants
| Cluster size | MNI coordinates | ||||||
|---|---|---|---|---|---|---|---|
| (voxels) | x | y | z | peakT | P | R/L | Region |
| WC | |||||||
| 6,588 | 66 | −28.5 | −25.5 | 9.554 | <0.001 | R | Inferior temporal gyrus |
| 69 | −34.5 | −16.5 | 8.280 | <0.001 | R | Middle temporal gyrus | |
| 60 | −13.5 | −36 | 7.582 | <0.001 | R | Inferior temporal gyrus | |
| 3,124 | −61.5 | −22.5 | −30 | 9.284 | <0.001 | L | Fusiform gyrus |
| −67.5 | −21 | −19.5 | 8.385 | <0.001 | L | Inferior temporal gyrus | |
| −55.5 | −9 | −39 | 8.204 | <0.001 | L | Inferior temporal gyrus | |
| 5,448 | −1.5 | −19.5 | −6 | 9.277 | <0.001 | L | Red nucleus |
| −3 | −27 | 9 | 6.323 | <0.001 | L | Thalamus | |
| −12 | −13.5 | 16.5 | 5.705 | <0.001 | L | Thalamus | |
| 5,983 | −3 | 49.5 | −25.5 | 8.049 | <0.001 | L | Medial frontal gyrus |
| −12 | 45 | −22.5 | 7.584 | <0.001 | L | Medial frontal gyrus | |
| 30 | 48 | −7.5 | 6.423 | <0.001 | R | Superior frontal gyrus | |
| 10,027 | −3 | −69 | −46.5 | 7.808 | <0.001 | L | Inferior semi–lunar lobule |
| 13.5 | −69 | −49.5 | 7.299 | <0.001 | R | Inferior semi‐lunar lobule | |
| 13.5 | −60 | −51 | 6.858 | <0.001 | R | Cerebellar tonsil | |
| 6,016 | 58.5 | −1.5 | 33 | 7.412 | <0.001 | R | Precentral gyrus |
| 54 | 13.5 | 27 | 7.047 | <0.001 | R | Inferior frontal gyrus | |
| 60 | −9 | 12 | 6.997 | <0.001 | R | Postcentral gyrus | |
| 4,837 | −4.5 | −84 | −4.5 | 6.649 | <0.001 | L | Lingual gyrus |
| −3 | −70.5 | 18 | 6.006 | <0.001 | L | Cuneus | |
| −4.5 | −63 | 21 | 5.997 | <0.001 | L | Posterior cingulate | |
| 571 | −13.5 | 49.5 | 15 | 6.398 | <0.001 | L | Medial frontal gyrus |
| −22.5 | 39 | 25.5 | 5.029 | 0.007 | L | Medial frontal gyrus | |
| 2,671 | −61.5 | −22.5 | 15 | 6.326 | <0.001 | L | Postcentral gyrus |
| −63 | −34.5 | 36 | 6.080 | <0.001 | L | Inferior parietal lobule | |
| −48 | −19.5 | 36 | 6.038 | <0.001 | L | Postcentral gyrus | |
| 77 | 33 | 40.5 | 13.5 | 6.050 | <0.001 | R | Medial frontal gyrus |
| 861 | −4.5 | −31.5 | 39 | 6.041 | <0.001 | L | Cingulate gyrus |
| −9 | −16.5 | 33 | 5.731 | <0.001 | L | Cingulate gyrus | |
| 9 | −12 | 36 | 5.486 | 0.001 | R | Cingulate gyrus | |
| 498 | 21 | −72 | −12 | 5.749 | <0.001 | R | Posterior lobe |
| 30 | −78 | −13.5 | 5.452 | 0.001 | R | Posterior lobe | |
| 113 | −19.5 | −24 | −19.5 | 5.363 | 0.001 | L | Parahippocampal gyrus |
| 55 | 4.5 | −12 | 25.5 | 5.300 | 0.002 | R | Cingulate gyrus |
| 90 | −48 | −37.5 | 39 | 5.087 | 0.005 | L | Supramarginal gyrus |
| 89 | 10.5 | −51 | −24 | 5.053 | 0.006 | R | Anterior lobe |
| 4.5 | −45 | −27 | 4.648 | 0.033 | R | Anterior lobe | |
| 122 | −13.5 | −51 | −25.5 | 5.012 | 0.007 | L | Anterior lobe |
| 151 | 31.5 | 24 | 3 | 4.925 | 0.010 | R | Claustrum |
| 62 | −58.5 | −58.5 | 18 | 4.841 | 0.015 | L | Superior temporal gyrus |
| BMI | |||||||
| 34,647 | −3 | −19.5 | −6 | 9.593 | <0.001 | L | Red nucleus |
| −63 | −22.5 | −28.5 | 8.979 | <0.001 | L | Fusiform gyrus | |
| −67.5 | −21 | −19.5 | 8.735 | <0.001 | L | Inferior temporal gyrus | |
| 11,421 | 66 | −31.5 | −24 | 9.036 | <0.001 | R | Inferior temporal gyrus |
| 69 | −39 | −13.5 | 8.388 | <0.001 | R | Middle temporal gyrus | |
| 31.5 | 15 | −42 | 8.060 | <0.001 | R | Superior temporal gyrus | |
| 2,130 | −12 | 46.5 | −24 | 7.230 | <0.001 | L | Medial frontal gyrus |
| −3 | 48 | −25.5 | 7.179 | <0.001 | L | Medial frontal gyrus | |
| −30 | 45 | −19.5 | 5.905 | <0.001 | L | Middle frontal gyrus | |
| 668 | −13.5 | 49.5 | 15 | 6.572 | <0.001 | L | Medial frontal gyrus |
| −7.5 | 52.5 | 22.5 | 5.853 | <0.001 | L | Medial frontal gyrus | |
| 3,055 | 43.5 | 37.5 | −15 | 6.194 | <0.001 | R | Inferior frontal gyrus |
| 19.5 | 52.5 | 0 | 6.094 | <0.001 | R | Medial frontal gyrus | |
| 12 | 58.5 | 7.5 | 5.945 | <0.001 | R | Medial frontal gyrus | |
| 931 | −4.5 | −31.5 | 39 | 6.036 | <0.001 | L | Cingulate gyrus |
| −9 | −18 | 33 | 5.541 | 0.001 | L | Cingulate gyrus | |
| 6 | −34.5 | 39 | 5.430 | 0.001 | R | Cingulate gyrus | |
| 78 | 21 | 46.5 | 19.5 | 5.635 | <0.001 | R | Medial frontal gyrus |
| 34 | 39 | −73.5 | 4.5 | 5.545 | 0.001 | R | Inferior occipital gyrus |
| 61 | −52.5 | 19.5 | 0 | 5.532 | 0.001 | L | Precentral gyrus |
| 109 | 16.5 | 42 | 19.5 | 5.524 | 0.001 | R | Anterior cingulate |
| 10.5 | 33 | 28.5 | 4.896 | 0.012 | R | Cingulate gyrus | |
| 39 | 31.5 | 42 | 12 | 5.488 | 0.001 | R | Middle frontal gyrus |
| 79 | −30 | −85.5 | 10.5 | 5.379 | 0.001 | L | Middle occipital gyrus |
| 71 | −48 | −37.5 | 39 | 5.173 | 0.003 | L | Supramarginal gyrus |
| −49.5 | −45 | 43.5 | 4.821 | 0.016 | L | Inferior parietal lobule | |
| 70 | −28.5 | 3 | −48 | 5.120 | 0.004 | L | Superior temporal gyrus |
| −28.5 | 10.5 | −45 | 4.789 | 0.018 | L | Superior temporal gyrus | |
| 76 | 60 | −58.5 | 25.5 | 5.085 | 0.005 | R | Superior temporal gyrus |
| 40 | 36 | −79.5 | 13.5 | 5.016 | 0.007 | R | Middle occipital gyrus |
| 35 | −22.5 | 40.5 | 25.5 | 4.961 | 0.009 | L | Medial frontal gyrus |
BMI, body mass index; L, left; MNI, Montreal Neurological Institute; R, right; WC, waist circumference.
Figure 1.
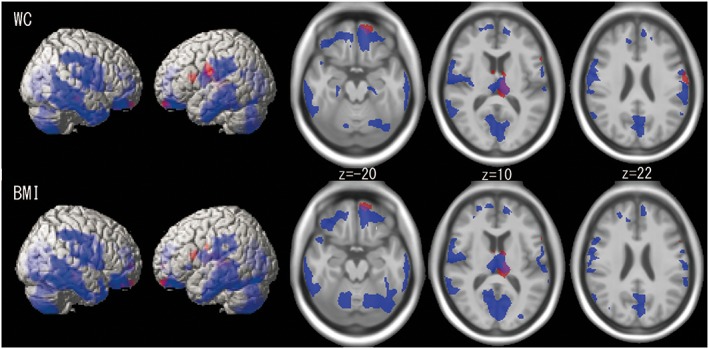
Lateral and axial images of the extent of grey matter regions that show negative correlations with waist circumference (WC) or body mass index (BMI) in male (blue) and female (red) participants. Overlapping areas are indicated in purple. The regions are almost identical for WC and BMI and are more widespread in male than in female participants.
Table 4.
MNI coordinates and grey matter regions negatively correlated with WC or BMI in female participants
| Cluster size | MNI coordinates | ||||||
|---|---|---|---|---|---|---|---|
| (voxels) | x | y | z | peakT | P | R/L | Region |
| WC | |||||||
| 1,380 | −10.5 | −12 | 18 | 6.317 | <0.001 | L | Thalamus |
| 3 | −15 | −7.5 | 6.032 | <0.001 | R | Red nucleus | |
| −4.5 | −33 | 10.5 | 5.897 | <0.001 | L | Thalamus | |
| 349 | −60 | −4.5 | 28.5 | 5.890 | <0.001 | L | Precentral gyrus |
| 111 | −57 | 12 | 19.5 | 5.739 | <0.001 | L | Inferior frontal gyrus |
| 253 | −7.5 | 58.5 | −22.5 | 5.595 | 0.001 | L | Medial frontal gyrus |
| −16.5 | 55.5 | −21 | 5.490 | 0.001 | L | Medial frontal gyrus | |
| 124 | 40.5 | −22.5 | −12 | 5.380 | 0.002 | R | Hippocampus |
| 87 | 7.5 | 37.5 | −30 | 5.166 | 0.006 | R | Rectal gyrus |
| 54 | 6 | 0 | 10.5 | 5.165 | 0.006 | R | Thalamus |
| BMI | |||||||
| 1,601 | −12 | −13.5 | 16.5 | 6.377 | <0.001 | L | Thalamus |
| 4.5 | −16.5 | −7.5 | 6.350 | <0.001 | R | Red nucleus | |
| −4.5 | −18 | −4.5 | 6.299 | <0.001 | L | Thalamus | |
| 79 | −57 | 12 | 19.5 | 5.877 | <0.001 | L | Inferior frontal gyrus |
| 238 | −7.5 | 58.5 | −22.5 | 5.633 | 0.001 | L | Medial frontal gyrus |
| −13.5 | 48 | −24 | 4.807 | 0.025 | L | Superior frontal gyrus | |
| 84 | 10.5 | 57 | 1.5 | 5.204 | 0.005 | R | Medial frontal gyrus |
| 48 | 15 | 43.5 | −22.5 | 5.177 | 0.005 | R | Medial frontal gyrus |
| 32 | −60 | −4.5 | 28.5 | 5.045 | 0.009 | L | Precentral gyrus |
| 54 | 33 | −22.5 | −10.5 | 4.844 | 0.021 | R | Hippocampus |
BMI, body mass index; L, left; MNI, Montreal Neurological Institute; R, right; WC, waist circumference.
For both male and female participants, neither the correlation between regional grey matter volume and WC nor that between regional grey matter volume and BMI was positive.
Discussion
The relationships of grey matter volume to WC and BMI in a large number of Japanese adults were evaluated. As for global grey matter volume, neither the relationship between GMF and WC nor that between GMF and BMI was significant. However, both WC and BMI were negatively correlated with regional grey matter volume in several structures. These regions were more widespread in men than in women.
As hypothesized, the participants in this study were classified as less obese than in previous studies: compared with the mean BMI reported in previous studies (25.02 ± 4.13 10, 28 ± 5 21 and 27.4 ± 4.5 or 27.2 ± 4.4 22), the mean BMI in this study was relatively low (24.7 ± 3.1 or 22.0 ± 3.3 for men or women, respectively, shown in Table 1) and classified as normal by WHO criteria 16. The percentage of participants with obesity with BMI of at least 30 was 4% for men and 3% for women, which are lower than the general prevalence of obesity in Europe and the USA (10–20%) 23. This may be one of the reasons why an association between global grey matter volume and WC or BMI was not seen in the present study. The participants in the report by Taki et al. 12 were Japanese, and their BMI was as low as that in this study (23.41 ± 3.00 for men and 22.23 ± 2.97 for women): they reported negative correlations between BMI and global grey matter volume in male but not female subjects among 1,428 participants. Their larger number of participants may account for this difference. Another explanation could be the use of self‐report data instead of measured data. As they have discussed, Taki et al. obtained data on height and weight by self‐questionnaire, and their study population might contain more people with overweight or obesity because subjects with higher BMIs significantly underestimated their weights, compared with those with smaller BMIs 12. Regarding this reporting bias, data of this study obtained by actually measuring height and weight may have estimated more precisely the correlations between BMI and global grey matter volume. The results of this study suggest that mild obesity in individuals with slightly high values of WC and BMI may influence regional grey matter volumes, even when these influences were not reflected in global grey matter volume.
The candidate mechanisms for obesity‐related differences in brain volume are excess body fat‐induced vascular abnormalities 28 and metabolic disorders (such as diabetes mellitus) 19, 20, both of which cause brain ischaemia, which in turn lead to brain atrophy. Together with previous studies 10, 12, 22, the present study suggests that several brain regions are affected by obesity: the bilateral frontal cortex, temporal cortex, inferior parietal cortex and medial occipital cortex, as well as bilateral cerebellar and midbrain thalamic regions. In female participants, the regions were more restricted to the frontal and left thalamic regions. Importantly, the regions that were affected in both male and female participants may be involved in obesity. Several investigators have proposed that the frontal regions are key to the regulation of taste, reward and behavioural control 11, 29. The thalamic region is one of the areas thought to be involved in motivational processes, along with the anterior cingulate cortex, caudate nucleus, putamen, hippocampus, hypothalamus, insula and medial prefrontal cortex 30.
Waist circumference and BMI were highly correlated, and the regional grey matter areas associated with each overlapped. However, in female participants, the total area of the regions correlated with WC was slightly greater than that of regions correlated with BMI. Some studies have reported that WC is a more sensitive than BMI as an indicator of differences in brain structure 10, 31. As in this study, Kurth et al. 10 found that the area of grey matter reduction associated with increases in WC was greater than that associated with increases in BMI when examined in female participants separately. Consistent with previous work, findings of this study suggest that WC is an effective index of risk for neurodegenerative disorders related to obesity, especially in populations with less obesity, such as the female participants of this study.
A sex‐associated difference exists in body fat deposition 32, 33. Indeed, sex‐dependent influences of obesity on brain volume have been reported in several studies 10, 12, 34. The present study suggests that sex influences the pattern of structure in the brain associated with body fat and that men are more susceptible to obesity‐related differences in brain volume. Both WC and BMI in the current study were larger in men than in women. Therefore, female participants with WC or BMI levels comparable with those in men may show patterns of regional grey matter volume difference similar to those in men: a future study in a more homogeneous group is needed to examine this possibility.
In summary, this finding of low regional grey matter volume associated with high WC and BMI in community‐dwelling adults suggests that even mild obesity can affect regional brain structures. Although future studies will be needed to confirm whether the relationship between mild obesity and brain volume is quantitative or qualitative (i.e. ethnicity‐specific), this finding suggests that interventions to people with mild obesity should be offered because, like people with obesity, they too may potentially be at risk for future declines in brain function.
Conflict of Interest Statement
The authors declare no conflicts of interest.
Acknowledgements
This work was funded by the ImPACT Program of Council for Science, Technology and Innovation (Cabinet Office, Government of Japan) and was supported in part by a High Technology Research Center Grant from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (MEXT) and MEXT‐Supported Program for the Strategic Research Foundation at Private Universities, 2014–2018.
Hayakawa, Y. K. , Sasaki, H. , Takao, H. , Yoshikawa, T. , Hayashi, N. , Mori, H. , Kunimatsu, A. , Aoki, S. , and Ohtomo, K. (2018) The relationship of waist circumference and body mass index to grey matter volume in community dwelling adults with mild obesity. Obesity Science & Practice, 4: 97–105. doi: 10.1002/osp4.145.
References
- 1. Kivipelto M, Ngandu T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol 2005; 62: 1556–1560. [DOI] [PubMed] [Google Scholar]
- 2. Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18‐year follow‐up of overweight and risk of Alzheimer disease. Arch Intern Med 2003; 163: 1524–1528. [DOI] [PubMed] [Google Scholar]
- 3. Razay G, Vreugdenhil A. Obesity in middle age and future risk of dementia: midlife obesity increases risk of future dementia. BMJ 2005; 331: 455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Colin Bell A, Adair LS, Popkin BM. Ethnic differences in the association between body mass index and hypertension. Am J Epidemiol 2002; 155: 346–353. [DOI] [PubMed] [Google Scholar]
- 5. Sakurai M, Miura K, Takamura T, et al. Gender differences in the association between anthropometric indices of obesity and blood pressure in Japanese. Hypertens Res 2006; 29: 75–80. [DOI] [PubMed] [Google Scholar]
- 6. Jousilahti P, Tuomilehto J, Vartiainen E, Pekkanen J, Puska P. Body weight, cardiovascular risk factors, and coronary mortality. 15‐year follow‐up of middle‐aged men and women in eastern Finland. Circulation 1996; 93: 1372–1379. [DOI] [PubMed] [Google Scholar]
- 7. Ahima RS, Lazar MA. Physiology. The health risk of obesity – better metrics imperative. Science 2013; 341: 856–858. [DOI] [PubMed] [Google Scholar]
- 8. Malnick SD, Knobler H. The medical complications of obesity. QJM 2006; 99: 565–579. [DOI] [PubMed] [Google Scholar]
- 9. Gustafson DR, Steen B, Skoog I. Body mass index and white matter lesions in elderly women. An 18‐year longitudinal study. Int Psychogeriatr 2008; 16: 327–336. [DOI] [PubMed] [Google Scholar]
- 10. Kurth F, Levitt JG, Phillips OR, et al. Relationships between gray matter, body mass index, and waist circumference in healthy adults. Hum Brain Mapp 2013; 34: 1737–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pannacciulli N, Del Parigi A, Chen K, Le DS, Reiman EM, Tataranni PA. Brain abnormalities in human obesity: A voxel‐based morphometric study. NeuroImage 2006; 31: 1419–1425. [DOI] [PubMed] [Google Scholar]
- 12. Taki Y, Kinomura S, Sato K, et al. Relationship between body mass index and gray matter volume in 1,428 healthy individuals. Obesity 2008; 16: 119–124. [DOI] [PubMed] [Google Scholar]
- 13. Raji CA, Ho AJ, Parikshak NN, et al. Brain structure and obesity. Hum Brain Mapp 2010; 31: 353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sahakyan KR, Somers VK, Rodriguez‐Escudero JP, et al. Normal‐weight central obesity: implications for total and cardiovascular mortality. Ann Int Med 2015; 163: 827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Krakauer NY, Krakauer JC. A new body shape index predicts mortality hazard independently of body mass index. PLoS One 2012: e39504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. World Health Organization . Obesity: preventing and managing the global endemic, WHO Technical Report Series No. 894 WHO: Geneva, 2000. [PubMed]
- 17. Shiwaku K, Anuurad E, Enkhmaa B, et al. Overweight Japanese with body mass indexes of 23.0–24.9 have higher risks for obesity‐associated disorders: a comparison of Japanese and Mongolians. Int J Obes Relat Metab Disord 2004; 28: 152–158. [DOI] [PubMed] [Google Scholar]
- 18. Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity‐related health risk. Am J Clin Nutr 2004; 79: 379–384. [DOI] [PubMed] [Google Scholar]
- 19. Zhu S, Heshka S, Wang Z, et al. Combination of BMI and waist circumference for identifying cardiovascular risk factors in whites. Obes Res 2004; 12: 633–645. [DOI] [PubMed] [Google Scholar]
- 20. Dalton M, Cameron AJ, Zimmet PZ, et al. Waist circumference, waist‐hip ratio and body mass index and their correlation with cardiovascular disease risk factors in Australian adults. J Intern Med 2003; 254: 555–563. [DOI] [PubMed] [Google Scholar]
- 21. Debette S, Beiser A, Hoffmann U, et al. Visceral fat is associated with lower brain volume in healthy middle‐aged adults. Ann Neurol 2010; 68: 136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Janowitz D, Wittfeld K, Terock J, et al. Association between waist circumference and gray matter volume in 2344 individuals from two adult community‐based samples. NeuroImage 2015; 122: 149–157. [DOI] [PubMed] [Google Scholar]
- 23. The examination committee of criteria for ‘obesity disease’ in Japan, Japan Society for the Study of Obesity. New criteria for ‘obesity disease’ in Japan. Circ J 2002; 66: 987–992. [DOI] [PubMed] [Google Scholar]
- 24. Rolls BJ, Fedoroff IC, Guthrie JF. Gender differences in eating behavior and body weight regulation. Health Psychol 1991; 10: 133–142. [DOI] [PubMed] [Google Scholar]
- 25. Examination committee of criteria for metabolic syndrome in Japan. Criteria for metabolic syndrome in Japan. J Jpn Soc Intern Med 2005; 94: 188–203. [in Japanese]. [Google Scholar]
- 26. Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 27. Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol 1987; 149: 351–356. [DOI] [PubMed] [Google Scholar]
- 28. Sorisky A. Molecular links between obesity and cardiovascular disease. Am J Ther 2002; 9: 516–521. [DOI] [PubMed] [Google Scholar]
- 29. Walther A, Birdsill AC, Glisky EL, Ryan L. Structural brain differences and cognitive functioning related to body mass index in older females. Hum Brain Mapp 2010; 31: 1052–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wallner‐Liebmann S, Koschutnig K, Reishofer G, et al. Insulin and hippocampus activation in response to images of high‐calorie food in normal weight and obese adolescents. Obesity 2010; 18: 1552–1557. [DOI] [PubMed] [Google Scholar]
- 31. Gwozdz W, Sousa‐Poza A, Reisch LA, et al. Peer effects on obesity in a sample of European children. Econ Hum Biol 2015; 18: 139–152. [DOI] [PubMed] [Google Scholar]
- 32. Goodman‐Gruen D, Barrett‐Connor E. Sex differences in body fat and body fat distribution in the elderly. Am J Epidemiol 1996; 143: 898–906. [DOI] [PubMed] [Google Scholar]
- 33. Lovejoy JC, Sainsbury A. Stock Conference 2008 Working Group. Sex differences in obesity and the regulation of energy homeostasis. Obes Rev 2009; 10: 154–167. [DOI] [PubMed] [Google Scholar]
- 34. Mueller K, Anwander A, Möller HE, et al. Sex‐dependent influences of obesity on cerebral white matter investigated by diffusion‐tensor imaging. PLoS One 2011; 6: e18544. [DOI] [PMC free article] [PubMed] [Google Scholar]


