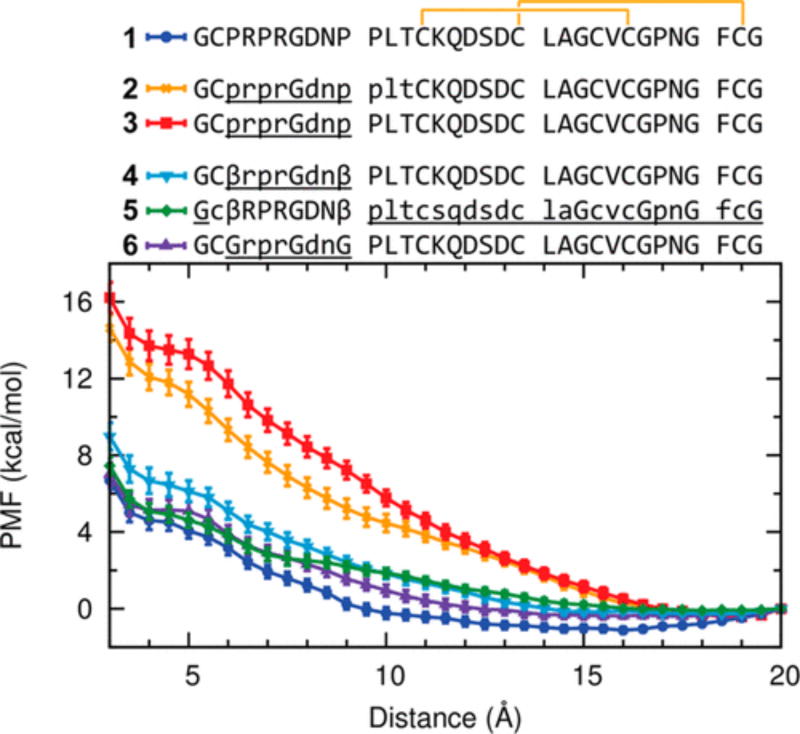
Figure 6.
Heterochiral EETI-II 2.5F proteins containing β-Ala and Gly substitutions require less free energy to fold. Steered molecular dynamics (SMD) simulations of homochiral EETI-II 2.5F (1) and several heterochiral EETI-II 2.5F analogues without (2 and 3) and with (4–6) β-Ala or Gly substitutions. Potentials of mean force (PMF) as a function of the distance between the two Sγ atoms of residues Cys2 and Cys24. For each system, the 100 SMD trajectories were separated into 20 groups with five trajectories in each group. The PMF for each group was estimated using Jarzynski’s equality. The results from the 20 groups were then averaged and error bars (standard errors of mean) calculated.