Abstract
Canadian Animal Health Institute (CAHI) data are used for provincial, national, and international comparisons of Canadian animal antimicrobial use. The objectives of this paper were to: i) use CAHI and British Columbia (BC) antimicrobial distribution data to group BC antimicrobial sales into the following BC retail distribution channels: over-the-counter retail outlets, livestock and poultry feed mills, aquaculture feed mills, livestock and poultry veterinarians, and companion animal veterinarians; and ii) to validate the CAHI BC distribution data and BC’s antimicrobial distribution data from 2012 to 2014. Annual total antimicrobial distribution and distribution by antimicrobial class were presented for each distribution channel. The distribution of medically important antimicrobials for production animals was validated, the distribution of ionophores was not. A lack of data precluded any attempt to validate the distribution of antimicrobials for companion animals. Each distribution channel typically experienced substantial fluctuations in total antimicrobial use and use by antibiotic class at least once over the 3-year period. The validated data are useful for evidence-based analysis of a proposed Canadian policy requiring a veterinary prescription for all medically important antimicrobials.
Résumé
Distribution des antimicrobiens pour animaux en Colombie-Britannique pour la vente de médicaments en vente libre et par des vétérinaires, de 2012 à 2014. Les données de l’Institut canadien de la santé animale (ICSA) sont utilisées pour effectuer des comparaisons provinciales, nationales et internationales de l’utilisation des antimicrobiens chez les animaux au Canada. Cet article avait pour objectifs : i) d’utiliser les données de distribution des antimicrobiens de l’ICSA et de la Colombie-Britannique afin de regrouper les ventes d’antimicrobiens en Colombie-Britannique (C.-B.) selon les réseaux de distribution au détail suivants en C.-B. : les commerces de vente libre, les usines d’aliments pour le bétail et la volaille, les usines d’aliments pour l’aquaculture, les vétérinaires pour le bétail et la volaille et les vétérinaires pour animaux de compagnie; et ii) de valider les données de distribution de l’ICSA en C.-B. ainsi que les données de distribution des antimicrobiens de la C.-B. de 2012 à 2014. La distribution totale annuelle des antimicrobiens et la distribution selon la catégorie d’antimicrobiens ont été présentées selon chaque réseau de distribution. La distribution des antimicrobiens importants sur le plan médical pour les animaux de production a été validée, contrairement à la distribution des ionophores. Une absence de données a empêché toute tentative de valider la distribution des antimicrobiens pour les animaux de compagnie. Chaque réseau de distribution a typiquement présenté des fluctuations substantielles dans l’utilisation totale des antimicrobiens et l’utilisation selon la catégorie d’antibiotique au moins une fois au cours de la période de 3 ans. Les données validées sont utiles pour l’analyse factuelle d’une politique canadienne proposée exigeant une prescription vétérinaire pour tous les antimicrobiens importants sur le plan médical.
(Traduit par Isabelle Vallières)
Introduction
Antimicrobial use (AMU) in animals is of considerable interest due to the global concern with antimicrobial resistance in human pathogens. The interest includes use in the various animal species and sales by distribution channel (i.e., sales by veterinarians versus over-the-counter sales). Canadian federal legislation requires a prescription for the sale of some antimicrobials and not others. The latter are known as over-the-counter (OTC) products. The Canadian government has proposed requiring a prescription (1) for all medically important antimicrobials (2) used in animals. However, there is a lack of information on Canadian antimicrobial sales by distribution channel. Filling this information gap could be useful in evidence-based analysis of an expanded veterinary prescription requirement and other AMU policies.
In Canada, the Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS) is the primary source of animal AMU surveillance data. Antimicrobial use for broilers and grower-finisher hogs (3) is included in CIPARS, as are data from the Canadian Animal Health Institute (CAHI) on quantities of antimicrobials distributed for sale for use in animals. The CAHI data have been used in Canada’s international comparisons of AMU. The CAHI is the trade association representing companies that manufacture and distribute pharmaceuticals for administration to animals. As of 2012, CAHI’s total annual distributions have been broken down by province, and by use in companion animals versus production animals (4). The World Health Organization (WHO) states that the accuracy of such sales data must be assessed (5). Weese et al (6) caution that the accuracy of voluntarily disclosed antimicrobial sales data provided by pharmaceutical companies, feed mills, and others may be poor. While Grave et al (7) note national and or regional sales data can be used to validate data obtained from other sources.
The government of British Columbia generates antimicrobial distribution data (8) from its licensing of over-the-counter (OTC) retailers and feed mills. The objectives of this paper were to: i) use the CAHI and BC licensing data sources to group BC antimicrobial data into the following 5 retail distribution channels: OTC retail outlets, livestock and poultry feed mills, aquaculture feed mills, livestock and poultry veterinarians, and companion animal veterinarians; and ii) to validate the CAHI BC production animal distribution data and the BC licensing data (BC LD).
Materials and methods
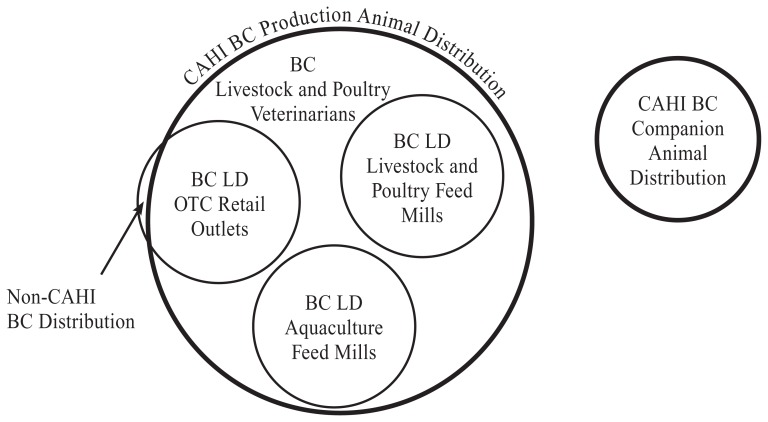
Grouping the BC retail distribution of antimicrobials into 5 distribution channels was accomplished using the CAHI and BC LD as illustrated in Figure 1. In this section, each data source is described, followed by the methods used to group the data. Then the methods to validate the CAHI data and BC LD are presented.
Figure 1.
The British Columbia licensing data (BC LD) and the Canadian Animal Health Institute (CAHI) data sources, and their relationships, used to group BC’s antimicrobial distribution into the following 5 retail distribution channels: BC LD over-the-counter (OTC) Retail Outlets, BC LD Livestock and Poultry Feed Mills, BC LD Aquaculture Feed Mills, BC Livestock and Poultry Veterinarians, and CAHI BC Companion Animal Distribution.
CAHI has estimated its members’ annual provincial distribution of antimicrobials for 2012 to 2014, measured in kg of active ingredients per antimicrobial class, for production and companion animals (3,4,9). The CAHI data include information from its member companies that manufacture, wholesale, and distribute antimicrobial products in Canada for use in animals. The CAHI data include sales to feed manufacturers, veterinary clinics, and OTC retail outlets. Estimates on CAHI members’ sales represent over 90% of all sales of licensed animal pharmaceuticals in Canada (3), but an estimate of CAHI’s members’ shares of total licensed animal antimicrobials is not available. Antimicrobial use was assigned to either production animals (inclusive of horses) or companion animals by the CAHI members according to label claim. In situations in which mixed species were indicated on the label, the manufacturer assigned (estimated) the species as either companion animal or production animal based on the veterinary clinic practice profile. The attribution of antimicrobials sold in each province to type of animal (companion animals versus production animals) (Figure 1) was based on multiplying a national average percentage of the antimicrobial sold for companion animals/production animals by the total reported in that province (3).
Briefly, the BC Ministry of Agriculture issues licences under the BC Veterinary Drugs Act and associated regulation for the sale of OTC veterinary drugs by livestock and poultry feed mills, aquaculture feed mills, and OTC retail outlets (Figure 1). Pharmacies and veterinarians can also sell OTC veterinary drugs and are exempt from the BC veterinary drugs legislation licensing requirements. As a condition of licensing, BC licensees annually submit licensing data (BC LD) to the Ministry. The BC LD varies with the distribution channel.
The BC LD from the livestock and poultry feed mills and the OTC retail outlets record the business’ veterinary drug purchases from wholesalers or distributors. The purchase records include the date of purchase, name of supplier, quantity purchased, the generic name, trade name, and name of the manufacturer of the drug. Aquaculture feeds medicated with antimicrobials require a veterinary prescription (10). The BC LD submitted to the Ministry by the aquaculture feed mills is the information on the aquaculture prescriptions, including the date of feed manufacture, production class of fish to be fed, proprietary and generic drug name, and concentration of active ingredient. Antimicrobial products from the BC LD are annually entered into an Excel spreadsheet (Microsoft Corporation, Redmond, Washington) for compilation and analysis (8). The spreadsheet was used to generate the annual retail antimicrobial distribution for BC LD OTC Retail Outlets, BC LD Livestock and Poultry Feed Mills, and BC LD Aquaculture Feed Mills (Figure 1), measured in kg of active ingredient.
The BC LD spreadsheet was analyzed to annually determine the antimicrobials that were not distributed by CAHI members, measured in kg of active ingredient. The CAHI membership was determined by reviewing the CAHI on-line membership list (11) and in consultation with Ms. Jean Szkotnicki, CAHI President (personal communication). The non-CAHI products were distributed only by BC LD OTC Retail Outlets (Figure 1). Most non-CAHI distributed OTC products were labelled for production animal use. A few products included label indications for production animals and companion animals, but such products annually accounted for less than 6% of the kg of active ingredients annually distributed by non-CAHI members. As BC’s licensed OTC retailers are essentially farm supply stores, all non-CAHI distributed products were assigned to production animal use.
The total wholesale distribution of antimicrobials into BC for production animals in a given year was calculated as (Figure 1):
| (Equation 1) |
The BC wholesale distribution of production animal antimicrobials was grouped into the following retail distribution channels: BC LD OTC Retail Outlets, BC LD Livestock and Poultry Feed Mills, BC LD Aquaculture Feed Mills, and BC Livestock and Poultry Veterinarians (Figure 1). The distribution by BC Livestock and Poultry Veterinarians was estimated by using the CAHI BC Production Animal Distribution data and the BC LD. Specifically, the annual distribution of antimicrobials by BC Livestock and Poultry Veterinarians was calculated as:
| (Equation 2) |
Review of the Compendium of Veterinary Products (12) did not identify any prescription antimicrobials distributed by non-CAHI members. Therefore, CAHI BC Companion Animal Distribution was deemed to represent BC companion animal veterinarians’ retail distribution of prescription antimicrobials (Figure 1).
Using Equations 1 and 2, BC’s annual wholesale production animal distribution and retail distribution of antimicrobials were calculated for 2012, 2013, and 2014. All antimicrobial classes reported by CAHI, with the exception of chemical coccidiostats which were not included in the BC data compilation, were analyzed using the CAHI identified active ingredients in each class. Nicarbazin was included in the CAHI ionophores antimicrobial class for 2012 and 2013 (4,9). For these years, BC feed mill purchases of nicarbazin, which is typically considered a chemical coccidiostat, was calculated using the feed mill purchase records.
Two approaches were used to validate the CAHI production animal distribution data and the BC LD (data from the BC OTC retail outlets, livestock and poultry feed mills, and aquaculture feed mills) and relied on the distribution by BC Livestock and Poultry Veterinarians being the difference in Equation 2. First, the data sources were validated if sales of each antimicrobial class by BC Livestock and Poultry Veterinarians was non-negative. A negative sale technically indicates product returns were greater than sales and this suggests an inconsistency between the CAHI and BC data. Second, although ionophores were primarily administered in feed, an oral bolus product was available for cattle. As British Columbia’s OTC retailers did not report sales of the bolus product, then sales were likely occurring through veterinarians. As a result, distribution of ionophores by BC Livestock and Poultry Veterinarians was expected to be greater than zero, and a non-positive value would invalidate the CAHI Production Animal Distribution data and the BC LD used to calculate the ionophore distribution.
Results
The BC animal antimicrobial distribution results for 2012, 2013, and 2014 are presented in Tables 1, 2, and 3, respectively. The antimicrobial classes and the active ingredients in each class are those reported by CIPARS (3,4,9). The active ingredients distributed OTC (i.e., by BC LD OTC Retail Outlets or BC LD Livestock and Poultry Feed Mills) are underlined.
Table 1.
2012 wholesale distribution of animal antimicrobials in BC parsed into retail distribution channels (kg).
| Antimicrobial classa | Active ingredientsa,b | BC production animal wholesale distribution | Retail distribution | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| BC LD OTC Retail Outlets | Feed Mills | Veterinarians | ||||||
|
|
|
|
||||||
| CAHI BC Production Animal | Non- CAHI BC Distribution | BC LD Livestock and Poultry (OTC) | BC LD Aquaculture (Rx) | BC Livestock and Poultryc | CAHI BC Companion Animal | |||
| Aminoglycosides | Amikacin, apramycin, dihydrostreptomycin, gentamicin, neomycin, spectinomycin, streptomycin | 597 | 10 | 626 | 10 | −29 | 1 | |
| β-Lactams and penicillin | Amoxicillin, ampicillin, clavulanic acid, cloxicillin, penicillin G | 9719 | 1433 | 1724 | 6562 | 247 | ||
| Cephalosporins | Cefaclor, cefadroxil, cefovecin, ceftiofur, cephapirin | 251 | 251 | 406 | ||||
| Fluoroquinolones | Enrofloxacin, danofloxacin, difloxacin, marbofloxacin, orbifloxacin | 20 | 20 | 22 | ||||
| Ionophores | Lasalocid, maduramicin, monensin, narasin, nicarbazin, salinomycin | 26 973 | 32 052 | −5079 | ||||
| Lincosamides | Clindamycin, lincomycin, pirlimycin | 81 | 22 | 59 | ||||
| Macrolides | Erythromycin, gamithromycin, tildipirosin, tilmicosin, tulathromycin, tylosin | 454 | 414 | 40 | ||||
| Other antimicrobials | Bacitracin, bambermycin, florfenicol, nitrofurantoin, nitrofurazone, novobiocin, ormethoprim, polymyxin, tiamulin, virginiamycin | 17 133 | 15 660d | 702e | 771 | 123 | ||
| Tetracyclines | Chlortetracycline, oxytetracycline, tetracycline | 15 233 | 12 | 610 | 4915 | 4764 | 4956 | |
| Trimethoprim and sulfonamides | Sulfabenzamide, sulfacetamide, sulfadiazine, sulfadimethoxine, sulfadoxine, sulfaguanidine, sulfamerazine, sulfamethazine, sulfanilamide, sulfaquinoxaline, sulfathiazole, trimethoprim | 2059 | 119f | 130f | 1142 | 106 | 800 | 41 |
| Total | 72 520 | 141 | 2799 | 55 939 | 5572 | 8351 | 840 | |
The antimicrobial classes and the active ingredients in each class are those reported by CIPARS (4).
Underlines denote sold in BC LD OTC Retail Outlets or BC LD Livestock and Poultry Feed Mills.
Distribution by BC Livestock and Poultry Veterinarians = CAHI BC Production Animal + Non-CAHI BC Distribution — BC LD OTC Retail Outlets — BC LD Livestock and Poultry Feed Mills — BC LD Aquaculture Feed Mills.
Consists of: bacitracin 13 679 kg; bambermycin 2 kg; tiamulin 71 kg; virginiamycin 1908 kg.
Florfenicol.
Includes succinylsulfathiazole.
Table 2.
2013 wholesale distribution of animal antimicrobials in BC parsed into retail distribution channels (kg).
| Antimicrobial classa | Active ingredientsa,b | BC Production animal wholesale distribution | Retail distribution | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| BC LD OTC Retail Outlets | Feed Mills | Veterinarians | ||||||
|
|
|
|
||||||
| CAHI BC Production Animal | Non- CAHI BC Distribution | BC LD Livestock and Poultry (OTC) | BC LD Aquaculture (Rx) | BC Livestock and Poultryc | CAHI BC Companion Animal | |||
| Aminoglycosides | Amikacin, apramycin, dihydrostreptomycin, gentamicin, neomycin, spectinomycin, streptomycin | 628 | 7 | 382 | 11 | 242 | ||
| β-Lactams and penicillin | Amoxicillin, ampicillin, cloxicillin, penicillin G, sulbactam | 10 539 | 717 | 2941 | 6881 | 129 | ||
| Cephalosporins | Cefaclor, cefadroxil, cefovecin, ceftiofur, cephapirin | 168 | 168 | 13 | ||||
| Fluoroquinolones | Enrofloxacin, danfloxacin, difloxacin, marbofloxacin, orbifloxacin | 34 | 34 | 15 | ||||
| Ionophores | Lasalocid, maduramicin, monensin, narasin, nicarbazin, salinomycin | 17 890 | 32 832 | −14 942 | ||||
| Lincosamides | Clindamycin, lincomycin, pirlimycin | 89 | 35 | 54 | ||||
| Macrolides | Erythromycin, gamithromycin, tildipirosin, tilmicosin, tulathromycin, tylosin, tylvalosin | 928 | 378 | 550 | ||||
| Other antimicrobials | Bacitracin, bambermycin, chloramphenicol, clavulanic acid, florfenicol, nitrofurantoin, nitrofurazone, novobiocin, ormethoprim, polymyxin, tiamulin, virginiamycin | 11 259 | 10 647d | 349e | 263 | 8 | ||
| Tetracyclines | Chlortetracycline, oxytetracycline, tetracycline | 12 474 | 13 | 397 | 4250 | 4625 | 3215 | |
| Trimethoprim and sulfonamides | Sulfabenzamide, sulfacetamide, sulfadiazine, sulfadimethoxine, sulfadoxine, sulfaguanidine, sulfamerazine, sulfamethazine, sulfanilamide, sulfaquinoxaline, sulfathiazole, trimethoprim | 2341 | 129f | 132f | 1007 | 125 | 1206 | 54 |
| Total | 56 350 | 149 | 1628 | 52 101 | 5099 | −2329 | 219 | |
The antimicrobial classes and the active ingredients in each class are those reported by CIPARS (9).
Underlines denote sold in BC LD OTC Retail Outlets or BC LD Livestock and Poultry Feed Mills.
Distribution by BC Livestock and Poultry Veterinarians = CAHI BC Production Animal + Non-CAHI BC Distribution — BC LD OTC Retail Outlets — BC LD Livestock and Poultry Feed Mills — BC LD Aquaculture Feed Mills.
Consists of: bacitracin 9287 kg; tiamulin 45 kg; virginiamycin 1316 kg.
Florfenicol.
Includes succinylsulfathiazole.
Table 3.
2014 wholesale distribution of animal antimicrobials in BC parsed into retail distribution channels (kg).
| Antimicrobial classa | Active ingredientsa,b | BC Production animal wholesale distribution | Retail distribution | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| BC LD OTC Retail Outlets | Feed Mills | Veterinarians | ||||||
|
|
|
|
||||||
| CAHI BC Production Animal | Non- CAHI BC Distribution | BC LD Livestock and Poultry (OTC) | BC LD Aquaculture (Rx) | BC Livestock and Poultryc | CAHI BC Companion Animal | |||
| Aminoglycosides | Amikacin, apramycin, dihydrostreptomycin, gentamicin, neomycin, spectinomycin, streptomycin | 614 | 4 | 502 | 7 | 109 | ||
| β-Lactams and penicillin | Amoxicillin, ampicillin, cloxicillin, penicillin G, sulbactam, clavulanic acid | 11 267 | 1 | 982 | 2970 | 7316 | 138 | |
| Cephalosporins | Ceftiofur, cephapirin, cefovecin, cefaclor, cefadroxil | 201 | 201 | 15 | ||||
| Fluoroquinolones | Enrofloxacin, difloxacin, marbofloxacin, orbifloxacin | 38 | 38 | 17 | ||||
| Ionophores | Lasalocid, maduramicin, monensin, salinomycin | 11 008 | 13 688 | −2680 | ||||
| Lincosamides | Clindamycin, lincomycin, pirlimycin | 92 | 27 | 65 | ||||
| Macrolides | Erythromycin, gamithromycin, tilmicosin, tylosin, tulathromycin, | 582 | 1 | 267 | 314 | |||
| Other antimicrobials | Avilamycin, bacitracin, bambermycin, chloramphenicol, florfenicol, nitrofurantoin, nitrofurazone, novobiocin, polymyxin, tiamulin, virginiamycin | 11 940 | 10 536d | 1211e | 193 | 9 | ||
| Tetracyclines | Chlortetracycline, oxytetracycline, tetracycline | 19 384 | 11 | 233 | 4882 | 4302 | 9978 | |
| Trimethoprim and sulfonamides | Ormethoprim, trimethoprim, sulfabenzamide, sulfacetamide, sulfadiazine, sulfadimethoxine, sulfadoxine, sulfaguanidine, sulfamerazine, sulfamethazine, sulfanilamide, sulfaquinoxaline, sulfathiazole | 2387 | 129f | 120f | 937 | 77 | 1382 | 55 |
| Total | 57 513 | 145 | 1838 | 33 314 | 5590 | 16 916 | 234 | |
The antimicrobial classes and the active ingredients in each class are those reported by CIPARS (3).
Underlines denote sold in BC LD OTC Retail Outlets or BC LD Livestock and Poultry Feed Mills.
Distribution by BC Livestock and Poultry Veterinarians = CAHI BC Production Animal + Non-CAHI BC Distribution — BC LD OTC Retail Outlets — BC LD Livestock and Poultry Feed Mills — BC LD Aquaculture Feed Mills.
Consists of: bacitracin 9350 kg; tiamulin 57 kg; virginiamycin 1129 kg.
Florfenicol.
Includes succinylsulfathiazole.
For each year, the calculated sales of each antimicrobial class by BC Livestock and Poultry Veterinarians were positive and validated the CAHI BC Production Animal Distribution data and the BC LD, with the exception of aminoglycoside distribution in 2012 and ionophore distribution in all 3 years. Distribution of aminoglycosides in 2012 was calculated as –29 kg, suggesting an inconsistency between the CAHI BC Production Animal Distribution data and the BC LD. This amount is less than 5% of the CAHI estimate of aminoglycoside use in production animals. The BC LD consistently reported distribution of a larger amount of ionophores than the CAHI data, and resulted in a negative calculated distribution by BC Livestock and Poultry Veterinarians. In the extreme, the 2013 purchases of ionophores by BC LD Livestock and Poultry Feed Mills was 184% of that reported by CAHI.
In each year, the non-CAHI BC Distribution of antimicrobials was small in absolute size (< 150 kg) and relative size (< 0.5% of CAHI distribution for production and companion animals). In each year, the majority of the non-CAHI BC distribution was sulfonamides and accounted for < 6% of the CAHI BC distribution (production animal and companion animal) of the trimethoprim and sulfonamides antimicrobial class.
On average, approximately 3/4 of the annual BC retail antimicrobial distribution was attributed to BC LD Livestock and Poultry Feed Mills from 2012 to 2014. Ionophores, which annually accounted for approximately half of this channel’s distribution, was the antimicrobial class with the greatest use, followed by other antimicrobials (i.e., bacitracin), tetracyclines, and β-lactams and penicillin (i.e., penicillin G).
British Columbia Livestock and Poultry Veterinarians annually distributed approximately12% of the total antimicrobial mass, and this channel’s share doubled if the negative calculated ionophore distribution was ignored. β-lactams and penicillin, and tetracyclines were the most commonly distributed antimicrobial classes in this channel, and represented over half of the active antimicrobial ingredients distributed by these veterinarians.
British Columbia LD Aquaculture Feed Mills accounted for slightly < 10% of the antimicrobials annually distributed in BC. Over 3/4 of this use was tetracyclines, followed by florfenicol.
Distribution by BC LD OTC Retail Outlets annually accounted for < 4% of the total distribution and most was penicillin G, followed by aminoglycosides, tetracyclines, and sulfonamides.
Approximately 1% of the BC distribution was by CAHI BC Companion Animal Veterinarians, and primarily consisted of β-lactams and penicillins, cephalosporins and other antimicrobials.
Over the 3-year period substantial fluctuations in total antimicrobial weight for each distribution channel were noted, with the exception of Non-CAHI BC Distribution and BC LD Aquaculture Feed Mills. In addition, over the 3-year period, each channel experienced a substantial fluctuation in the distribution of at least 1 antimicrobial class from one year to the next, with the exception of Non-CAHI BC Distribution. For example, CAHI BC Production Animal Distribution of macrolides more than doubled from 2012 to 2013. Penicillin G distributed by BC LD OTC Retail Outlets decreased approximately 50% from 2012 to 2013, while BC LD Livestock and Poultry Feed Mill distribution increased 71% over the same period. Annual aquaculture distribution of florfenicol decreased 50% then increased over 200% from 2012 to 2014. Distribution of tetracyclines by BC Livestock and Poultry Veterinarians more than tripled from 2013 to 2014. Cephalosporin and other antimicrobial distribution by CAHI BC Companion Animal Veterinarians decreased over 90% from 2012 to 2013.
The distribution channel with the greatest annual distribution of the individual antimicrobial classes varied. British Columbia LD OTC Retail Outlets and BC Livestock and Poultry Veterinarians had the greatest annual distribution of aminoglycosides, and β-lactams and penicillins, respectively. British Columbia Livestock and Poultry Veterinarians annually distributed the most cephalosporins and fluoroquinolones except for 2012 when companion animal veterinarians distributed more of each class. The primary distribution channel for ionophores was the BC LD Livestock and Poultry Feed Mills. British Columbia Livestock and Poultry Veterinarians annually distributed the greatest amount of lincosamides. The greatest distributer of macrolides was BC LD Livestock and Poultry Feed Mills in 2012, and BC Livestock and Poultry Veterinarians in 2013 and 2014. British Columbia LD Livestock and Poultry Feed Mills accounted for the greatest annual distribution of other antimicrobials. British Columbia Livestock and Poultry Veterinarians had the greatest distribution of tetracyclines in 2012 and 2014, while aquaculture mills distributed the most in 2013. The largest distributer of the trimethoprim and sulfonamides antimicrobial class was BC LD Livestock and Poultry Feed Mills in 2012 and BC Livestock and Poultry Veterinarians in 2013 and 2014.
Discussion
With the exception of the ionophores, the annual CAHI BC Production Animal Distribution data and the BC LD from 2012 to 2014 were validated. Consistency between the CAHI data and the BC LD was not unexpected as the data should largely represent opposite sides of the same transactions. That is, the CAHI Production Animal Distribution data were its members’ sales to BC retailers of production animal antimicrobials, and the BC LD for OTC retailers and livestock and poultry feed mills are those businesses’ purchases of antimicrobials from wholesalers or distributors. Furthermore, these 2 retail channels represented approximately 80% of the total provincial antimicrobial distribution. In contrast, the BC LD for aquaculture feed mills, which accounts for < 10% of the antimicrobials annually distributed in BC, represents prescription information which is expected to closely approximate retail sales of the antimicrobials. Retail antimicrobial sales may not equal wholesale sales due to changes in inventory. Validation of the CAHI BC Companion Animal Distribution of antimicrobials was precluded due to a lack of an alternative data source on BC companion animal veterinarians’ purchases or sales of antimicrobials.
There was a small discrepancy in the 2012 distribution of aminoglycosides. Explanations for this inconsistency include data recording errors, or discrepancies in how CAHI versus the BC LD allocate distribution to a given calendar year. Due to these reasons and others, CIPARS cautions (3) that annual data may be less reliable than data spanning multiple years. Although the validation of the annual production animal distribution data by antimicrobial class suggests they are reliable, each retail distribution channel had a large year-to-year fluctuation in at least one class, suggesting caution should be exercised when assessing annual changes in AMU. Specifically, caution should be exercised in extrapolating a single year-to-year change to a trend.
In addition to the fluctuations in distribution by antimicrobial classes, annual fluctuations in total antimicrobial sales for most of the retail distribution channels were noted. These fluctuations raise the issue of whether they were artifacts of data recording errors or true yearly variations in AMU. The fluctuations occurred in all data sources: the CAHI data (CAHI BC Companion Animal Distribution), the BC LD (BC LD OTC Retail Outlets, BC LD Livestock and Poultry Feed Mills, and the BC LD Aquaculture Feed Mills) and the distribution data that were a function of both the CAHI data and the BC LD (BC Livestock and Poultry Veterinarians).
The 2012 total national CAHI Companion Animal Distribution of the antimicrobial classes cephalosporins, other antimicrobials, and β-lactams and penicillin respectively decreased to approximately 3%, 7%, and 52% in 2013, with those lower levels continuing into 2014. Moreover, as exemplified by the CAHI BC Companion Animal Distribution data (Tables 1–3), each province experienced very similar respective patterns in the reported CAHI distribution of these 3 antimicrobial classes. The magnitude of declines and consistency across provinces is suggestive of a data recording error in the CAHI Companion Animal Distribution data. The minor annual changes in active ingredients associated with each antimicrobial class (Tables 1–3) were not expected to explain these patterns in distribution.
The retail distribution channels tend to be aggregates of the actions of many individuals, which made assessing the aggregate results difficult. In contrast, prescribing BC aquaculture veterinarians numbered less than 10. Informal discussions with some of these veterinarians suggested the fluctuations in florfenicol use in the BC LD Aquaculture Feed Mills were not unexpected with potential explanations including: changes in total production or production class of fish; changes in use of a given antimicrobial for a number of reasons including availability or changes in fish health status; and changes in prescribing practices such as altering the dose to ensure achievement of therapeutic levels. Differences in potency among antimicrobials could also have contributed to the fluctuations in kilograms of antimicrobials distributed by antimicrobial class or in total.
Fluctuations in AMU are not without precedent at the animal species level. Significant annual fluctuations in total AMU and use of some antimicrobial classes (measured in kg of active ingredient) have been noted in Canadian broilers (3) using farm level data. Fluctuations in total AMU and use of some antimicrobial classes (measured in kg of active ingredient) have been reported for Danish poultry and companion animals but not pigs or cattle using AMU data from a national prescription database (13,14). For example, a 58% increase in total AMU in Danish poultry (including broilers, layers, and turkeys) occurred in 2015 (15). Fluctuations in use (measured as defined daily doses animal at the farm level) of some antimicrobial classes have occurred in Dutch cattle, broilers, turkey, veal calves, and pigs with the latter also having fluctuations in total use (16). With the exception of the BC LD Aquaculture Feed Mills, the BC distribution channels were aggregations of data over multiple species and this is expected to dampen the AMU fluctuations of individual animal species, but the magnitude of the dampening is unknown.
The BC LD consistently reported significantly greater ionophore distribution than the CAHI data as reflected by the calculated negative ionophore sales by BC Livestock and Poultry Veterinarians (Tables 1 to 3). Furthermore, sustained release ionophore capsules were available for cattle and, although an OTC product, no licensed BC OTC retailers reported selling the product. Therefore, it was expected veterinarians were selling the product in which case the Livestock and Poultry Veterinarians’ sales of ionophores should have been positive. Due to the negative sales, the BC Production Animal Distribution data and BC LD on ionophore distribution could not be validated. The reason for the substantial discrepancy between the 2 AMU reporting systems is unclear. The BC LD and CAHI BC Production Animal Distribution data each exhibited moderate to substantial annual fluctuations in ionophore distribution, yet similar magnitudes of fluctuation in CAHI ionophore distribution were evident in approximately half of the other provinces over the same time period (3,4,9). CIPARS states there could be further interprovincial distribution of antimicrobials (3), but that seems unlikely to account for the 2 data sources’ large discrepancies in ionophore distribution.
Irregularities were noted in the CAHI assignment of antimicrobials to the chemical coccidiostats and ionophores, both of which are classes of coccidiostats. For example, nicarbazin, which is typically classified as a chemical coccidiostat, was included with the ionophores for 2012 and 2013 (4,9). In 2014, nicarbazin was classified as a chemical coccidiostat along with narasin (3), the latter is typically considered an ionophore. Excluding nicarbazin (data not shown) from the 2012 and 2013 BC LD Livestock and Poultry Feed Mill and including narasin (data not shown) in that distribution channel’s 2014 data resulted in BC Livestock and Poultry Veterinarians’ calculated retail distribution of ionophores being 4765 kg, and –3472 kg and –4310 kg in 2012, 2013, and 2014, respectively. These adjustments reduced the overall discrepancy in ionophore distribution between the CAHI BC Production Animal Distribution data and the BC LD, but the discrepancies remained sizable. The validation tests employed in this study were relatively weak so further validation of the CAHI BC Production Animal Distribution data and BC LD should be considered.
Perhaps the inability to validate the ionophore distribution data is unimportant because these antimicrobials are not considered to be medically important. Agunos et al (17), however, note the public health implications of ionophore use. The Government of Canada has proposed mandatory annual reporting (18) only of medically important antimicrobials for veterinary use. For each drug, the total quantity of antimicrobial sold or compounded with an estimate of the approximate quantity intended for each animal species must be reported by manufacturers and importers. The antimicrobial classes used in this study all contain medically important antimicrobials (2) except for the ionophores, and bambermycin which was included in the other antimicrobials class. This study validated the current voluntary reporting of medically important antimicrobials by antimicrobial class, but not by drug or animal species. The federal proposal does not mention reporting by province. Without provincial level data, future validation of the mandatory reported data would not be possible using the BC LD.
Due to a lack of data, this study did not include the distribution channels of antimicrobial importation by producers for their own use or the use, including importation, of active pharmaceutical ingredients. The latter would be captured by the proposed mandatory reporting requirement. The former would be eliminated for medically important antimicrobials under a new proposed federal regulation (18). However, the future oversight of antimicrobial purchases from internet pharmacies, including those not located in Canada, is unclear.
In BC, the CAHI distribution of antimicrobials represented over 99.5% of all antimicrobial sales, which is greater than CAHI’s estimated 90% market share of licensed animal pharmaceutical products (3). In BC, non-CAHI distribution of antimicrobials by OTC retailers is minor in terms of relative and absolute amount of active antimicrobial ingredients distributed. Non-CAHI distributed antimicrobials could also have been distributed by BC Livestock and Poultry Veterinarians or CAHI BC Companion Animal Veterinarians and this would have resulted in the underestimation of antimicrobial distribution by these channels. Due to a lack of data on antimicrobial distribution by BC veterinarians, the sizes of any underestimations are unknown.
Caution should be exercised in extrapolating the BC data on wholesale and retail antimicrobial distribution by channel to other provinces for a number of reasons including the interprovincial variability in animal demographics. For example, in 2014, BC ranked 1st among the provinces in farmed salmon receipts, 3rd in dairy, chicken, and turkey receipts, and 6th in hog and beef receipts (19). Yet, differences in national versus BC’s animal demographics apparently didn’t negatively impact the validation of the CAHI and BC antimicrobial distribution data. The CAHI provincial allocation of antimicrobial classes between companion and production animals relied on national averages of the percent sold to each of the 2 groups (3). Caution should be exercised in comparing the distribution among channels as the potency of antimicrobials is not considered, nor is the biomass of the underlying animal populations. For example, similar to the BC distribution data (Tables 1 to 3), the 2014 national kilograms of cephalosporins and fluoroquinolones distributed for production animals was greater than that for companion animals, yet companion animals had the greatest distribution when the biomass of the underlying animal populations was considered (20).
Validated AMU data can be useful for policy analysis. The current data, although lacking detail in use by antimicrobial or species of administration, can inform evidence-based analyses, including the proposed veterinary oversight requirement of veterinary prescription (1) for all medically important antimicrobials (2). For example, BC’s retail distribution data clarify that fluoroquinolones and cephalosporins, antimicrobials of greatest importance to human medicine (2), are distributed only by veterinarians and not OTC. Although this limited distribution might be obvious to some veterinarians, it might not be to others, including the general public or public health officials. Given fluoroquinolones and cephalosporins are not available OTC, a prescription use only policy for veterinary antimicrobials cannot be expected to reduce their use. However, if the policy results in producers newly employing a veterinarian to access antimicrobials, which are a cornerstone of animal welfare, it will result in potential new access to all veterinary prescription antimicrobials, including the fluroquinolones and cephalosporins. This is not to suggest an increased use of such important antimicrobials under veterinary oversight is imprudent. Nonetheless, this potential increased use of antimicrobials, which are of greatest importance to human medicine, is a non-intuitive outcome of a veterinary prescription use only policy with aims of improving stewardship.
The number of producers operating without veterinary oversight is unknown and the BC data should not be co-opted to address this knowledge gap. That is, OTC distribution is not equivalent to use without veterinary oversight. Producers operating under veterinary oversight could elect to purchase some OTC antimicrobials for a number of reasons including cost and accessibility.
Veterinary AMU surveillance goals tend to target highly detailed data which come at an increased cost. As evidenced by the current data, less costly and more aggregated data can also be useful in policy analysis and stewardship oversight.
Ironically, the planned AMU policy and regulatory changes will decrease the available AMU data in BC. For example, a prescription use only policy will eliminate antimicrobial distribution by the OTC retail outlet distribution channel and the associated AMU data source. Instead such products will likely be distributed through BC veterinarians, for whom there is currently no AMU distribution data. However, the federal regulatory and policy changes could present the opportunity to capture more detailed AMU data as currently occurs in BC with the collection of aquaculture prescription data. For example, the BC legislation on OTC veterinary drug retailers will likely require revision to be consistent with the proposed federal changes. This presents an opportunity to require more detailed AMU information from BC’s livestock and poultry feed mills, which is the retail distribution channel that accounts for approximately 75% of the province’s veterinary antimicrobials. These data would be nicely complemented by data on AMU prescribed by veterinarians and not administered in feed.
Acknowledgments
The thoughtful comments of 3 anonymous reviewers are gratefully acknowledged. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Government of Canada. Notice to Stakeholders: Update on Collaborative efforts to promote the prudent use of Medically-Important Antimicrobials (MIAs) in animals. Ottawa, Ontario: Health Canada; 2017. [Last accessed January 23, 2018]. Available from: https://www.canada.ca/en/health-canada/services/drugs-health-products/veterinary-drugs/antimicrobial-resistance/notice-stakeholders-update-collaborative-efforts-promote-prudentuse-medically-important-antimicrobials-animals.html. [Google Scholar]
- 2.Government of Canada. Categorization of Antimicrobial Drugs Based on Importance in Human Medicine. Ottawa, Ontario: Health Canada; 2009. [Last accessed January 23, 2018]. Available from: https://www.canada.ca/en/health-canada/services/drugs-health-products/veterinary-drugs/antimicrobial-resistance/categorization-antimicrobial-drugs-based-importance-human-medicine.html. [Google Scholar]
- 3.Government of Canada. Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS) 2014 Annual Report. Guelph, Ontario: Public Health Agency of Canada; 2016. [Google Scholar]
- 4.Government of Canada. Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS) 2012 Annual Report. Guelph, Ontario: Public Health Agency of Canada; 2014. [Last accessed January 23, 2018]. Available from: http://www.phac-aspc.gc.ca/cipars-picra/2012/annu-report-rapport-eng.php. [Google Scholar]
- 5.World Health Organization. Integrated surveillance of antimicrobial resistance: Guidance from a WHO Advisory Group. Geneva, Switzerland: 2013. [Last accessed January 23, 2018]. Available from: http://apps.who.int/iris/bitstream/10665/91778/1/9789241506311_eng.pdf?ua=1. [Google Scholar]
- 6.Weese JS, Page SW, Prescott JF. Chapter 7 Antimicrobial stewardship in animals. In: Giguere S, Prescrott JF, Dowling PM, editors. Antimicrobial Therapy in Veterinary Medicine. 5th ed. Ames, Iowa: John Wiley & Sons; 2013. pp. 117–132. [Google Scholar]
- 7.Grave K, Jensen VK, McEwen S, Kruse H. Monitoring of antimicrobial drug use in animals: Methods and applications. In: Aarestrup FM, editor. Antimicrobial Resistance in Bacteria of Animal Origin. Washington, DC: ASM Press; 2006. pp. 375–395. [Google Scholar]
- 8.Radke BR. Use of Over-the-Counter Antibiotics in BC Livestock and Poultry, 2002–2016. 2017. [Last accessed January 23, 2018]. Available from: http://www2.gov.bc.ca/assets/gov/farming-natural-resources-and-industry/agriculture-andseafood/animal-and-crops/agricultural-licenses-and-forms/bc_otc_amu_report_2002-2016.pdf.
- 9.Government of Canada. Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS) 2013 Annual Report. Guelph, Ontario: Public Health Agency of Canada; 2015. [Google Scholar]
- 10.Morrison DB, Saksida S. Trends in antimicrobial use in Marine Harvest Canada farmed salmon production in British Columbia (2003–2011) Can Vet J. 2013;54:1160–1163. [PMC free article] [PubMed] [Google Scholar]
- 11.Members. Canadian Animal Health Institute (CAHI); Guelph, Ontario: [Last accessed January 23, 2018]. Available from: http://www.cahi-icsa.ca/about/associate-members.aspx. [Google Scholar]
- 12.Inglis S, editor. Compendium of Veterinary Products. 25th Anniversary ed. (Canadian edition) Hensall, Ontario: North American Compendium; 2013. [Google Scholar]
- 13.DANMAP 2013 — Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. [Last accessed January 23, 2018]. Available from: http://www.danmap.org/~/media/Projekt%20sites/Danmap/DANMAP%20reports/DANMAP%202013/DANMAP%202013.ashx.
- 14.DANMAP 2012 — Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. [Last accessed January 23, 2018]. Available from: http://www.danmap.org/~/media/Projekt%20sites/Danmap/DANMAP%20reports/DANMAP%202012/Danmap_2012.ashx.
- 15.DANMAP 2015 — Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. [Last accessed January 23, 2018]. Available from: http://www.danmap.org/~/media/Projekt%20sites/Danmap/DANMAP%20reports/DANMAP%20%202015/DANMAP%202015.ashx.
- 16.Netherlands Veterinary Medicine Authority 2016. Usage of antibiotics in agricultural livestock in the Netherlands in 2015. Trends and benchmarking of livestock farms and veterinarians. [Last accessed January 23, 2018]. Available from: http://www.wur.nl/upload_mm/3/7/f/b17aafd6-2954-4ef2-8717-0adddc4be0bc_NethmapMaran2016_A.pdf.
- 17.Agunos A, Léger DF, Carson CA, et al. Antimicrobial use surveillance in broiler chicken flocks in Canada, 2013–2015. PLoS ONE. 2017;12:1–23. doi: 10.1371/journal.pone.0179384. Available from: http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0179384 Last accessed January 23, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Government of Canada. Regulations Amending the Food and Drug Regulations (Veterinary Drugs — Antimicrobial Resistance) [Last accessed January 23, 2018];Canada Gazette Part II: Official Regulations. 2017 May 17;151(10) Available from: http://www.gazette.gc.ca/rp-pr/p2/2017/2017-05-17/html/sor-dors76-eng.php. [Google Scholar]
- 19.British Columbia Ministry of Agriculture. Fast Stats 2014 British Columbia’s Agrifood and Seafood Sector. Dec, 2015. [Last accessed January 23, 2018]. Available from: http://www2.gov.bc.ca/assets/gov/farming-natural-resources-andindustry/agriculture-and-seafood/statistics/industry-and-sector-profiles/fast-stats/faststatsbc_2014.pdf.
- 20.Radke B. Letter to the Editor. Antimicrobial use in animals editorial — Comments on companion animal use and animal welfare. Can Vet J. 2016;57:1017. [PMC free article] [PubMed] [Google Scholar]