Abstract
There is a global mandate even in countries with low resources to improve the accuracy of testing biomarkers in breast cancer viz. oestrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2neu) given their critical impact in the management of patients. The steps taken include compulsory participation in an external quality assurance (EQA) programme, centralized testing, and regular performance audits for laboratories. This review addresses the status of ER/PR and HER2neu testing in India and possible reasons for the delay in development of guidelines and mandate for testing in the country. The chief cause of erroneous ER and PR testing in India continues to be easily correctable issues such as fixation and antigen retrieval, while for HER2neu testing, it is the use of low-cost non-validated antibodies and interpretative errors. These deficiencies can however, be rectified by (i) distributing the accountability and responsibility to surgeons and oncologist, (ii) certification of centres for testing in oncology, and (iii) initiation of a national EQA system (EQAS) programme that will help with economical solutions and identifying the centres of excellence and instill a system for reprimand of poorly performing laboratories.
Keywords: Biomarker, breast, cancer, HER2neu, oestrogen receptor, progesterone receptor, quality assurance
Introduction
The explosion of theranostics (a portmanteau of therapeutics and diagnostics) has led to an emergence of immunohistochemistry (IHC)-based predictive markers that are used to treat patients. The three biomarkers viz. oestrogen receptor/progesterone receptor (ER/PR) and HER2neu have markedly improved the prognosis of breast cancer with the use of appropriately targeted therapy, leading to the mandate of compulsory testing in all breast cancers and even in recurrences1,2,3. The emergence of additional therapies beyond tamoxifen and second-generation anti-HER2neu inhibitors has further intensified the interest in improving the accuracy of testing for these biomarkers4. IHC-based predictive markers are popular as these are cheaper, easy to implement and serve as a first screen to look for targets e.g. in breast cancer, only the equivocal (score 2) samples are reflex tested by fluorescent in situ hybridization (FISH) to confirm HER2neu amplification reducing the burden on testing laboratories. However, IHC has its unique set of flaws and the errors produced are frequently labelled as the black box of IHC testing5,6.
From the experience of large clinical trials, it is evident that there is a great variability in testing biomarkers in breast carcinoma which has prompted the American Society of Clinical Oncology (ASCO) and College of American Pathologists (CAP) (ASCO-CAP), National Comprehensive Cancer Network (NCCN) and individual countries (Spain, Sweden, Australia, Austria, etc.)1,2,3,7,8,9,10,11 to come out with the guidelines to ensure accurate testing. Following these guidelines is mandatory for testing laboratories in the most developed countries. In low-resource countries, though the guidelines for reporting are followed, the testing methodology is fallacious and cost is often cited as the chief cause for delay in implementation of good practices. This review addresses the present state of testing for ER/PR and HER2neu biomarkers (global versus India) and steps that are required to improve on it.
Learning from global experience
Globally, hormone receptor-positive cancers are the most common subtype of breast cancer, accounting for 78-80 per cent of all cases1,2,3,7,12,13. The global HER2neu IHC-based positivity rates range from 11 to 20 per cent2,3,14,15. The problem of erroneous results of ER/PR and HER2neu testing is universal and not confined to countries with low resources5. In a review of pathology testing procedures of patients enrolled in the ‘Breast International Group’ (BIG) I-98 trial, 73 of 105 (69%) ER-negative tumours were found to have more than 10 per cent positive cells and 66 of 6100 (1%) tumours locally reported ER positive were found to have no staining16. In an external quality assurance (EQA) programme involving 105 laboratories in Europe, reliable assays for ER and PR were found only in 24 (36%) of 66 laboratories participating in the continual EQA in spite of all centres having clinically validated assays17. In the Adjuvant Lapatinib and/or Trastuzumab Treatment Optimization trial, 4.3 per cent of tumours tested ER positive in local laboratories were found to be negative on central testing. More than 20 per cent of tumours were falsely reported as ER negative18.
Approximately 20 per cent of HER2neu assays performed at the primary treatment site's pathology department were incorrect when re-evaluated in a high-volume, central laboratory19,20,21. A false-negative diagnosis will deny potentially life-extending therapy to a truly HER2-positive patient. On the other hand, a false positive will result in exposure to a drug that has significant cardiotoxicity and exorbitant drug cost. High discordance rates between IHC and FISH are due to technical issues and should not be used to condemn the technique itself21. While the superiority of one method vs the other remains controversial, screening all cases with IHC and triaging selected cases for FISH testing is acceptable22,23.
The need for constant monitoring and EQA programme was felt two decades ago, and the United Kingdom National External Quality Assurance programme (UKNEQAS) was established nearly 30 yr ago (http://www.ukneqasiccish.org). UKNEQAS has published some seminal articles on improving ER and PR testing accuracy across the globe17,24,25. Proficiency testing for ER, PR and HER2neu was developed by the Royal College of Pathologists of Australasia Quality Assurance Program in 2001, and an audit on more than 8000 patients indicated that though overall the results for ER, PR and HER2neu fell within the established parameters, a number of individual laboratories did not meet the target values and the variation in results impacted patient treatment decisions10. In the widely known ‘Canadian disaster’, a false-negative index ER test report in Eastern Health led to investigation of the accuracy of ER testing in Newfoundland and Labrador and 40 per cent of over 2000 originally ER-negative cases were found to be ER positive on re-testing5,6,25,26. Although Canada has a national health care system, health care delivery is handled regionally, and Newfoundland had no regulatory body for accreditation or setting standards for conduct of clinical laboratory tests26. Within Canada, strong public reaction to the above event motivated a push for systemic changes in medical training and laboratory staffing26. Programme to regularly monitor ER/PR and HER2neu data from provinces was set up, and ER or PR positivity rates were monitored in several provinces27. The Canadian IHC Quality Control Programme subsequently evaluated 31 participating laboratories for ER/PR in 44 breast carcinomas and reported 100 per cent agreement when indeterminate results were excluded28. The Nordic Immunohistochemical Quality Control (NordiQC) documented that 20 per cent of the staining results in breast cancer IHC module were insufficient for diagnostic use29. Some of these EQA system (EQAS) programmes exercise more control and have a mandate for regulatory action against defaulting laboratories, e.g. UKNEQAS is required to notify the National Quality Assurance Advisory Panel of any cases of persistent poor performance in participating UK clinical laboratories (http://www.ukneqasiccish.org).
Even in the low-resource countries, steps are being taken to improve ER/PR and HER2neu testing. If the positivity rates are the judging ruler, one study from Africa documented an ER positivity of 72.8 per cent, PR in 64.8 and 17.6 per cent HER2 positivity similar to that observed in western countries30. The low per cent positivity in Asian countries may be at least in part due to testing issues. However, several studies from Philippines, Bangladesh, Vietnam and Malaysia have reported that with improved fixation and testing practices, the incidence of ER/PR is between 60 and 70 per cent1,31. In a study from the National Cancer Center in China, the ER/PR positivity rate was 78.4 and 79.7 per cent, respectively, while 25.5 per cent were HER2 positive32.
Recognizing the lacunae in testing methodology, Scientific Partnership for HER2 Testing Excellence (SPHERE) training programme (sponsored by Roche Pharma) was initiated in the Asia-Pacific region including 70 countries and supporting 120 laboratories for the UKNEQAS IHC and in situ hybridization (ISH) EQA programme33. A jump in the ‘pass rates’ for UKNEQAS programme, from 39 to 61 per cent was seen in HER2neu run across these countries (Dr Ibrahim Merdol, personal communication).
The Indian scene on breast cancer biomarker testing
One way of ensuring uniformity is to have data on the incidence of these biomarkers (ER/PR and HER2neu) for India to establish the minimum and maximum cut-offs. Data on biomarker prevalence in India are however, variable chiefly due to test-related issues. Most studies that reported lower hormone receptor positivity in patient population justified that our patient population was a decade younger than Western countries and had higher grade of tumours34,35. A summary of all studies published in this regard is given in Table including the results of our laboratory. The most valid hormone receptor positivity in Indian patient population reached between 60 and 70 per cent while rates for HER2neu positivity in breast cancer were between 20 and 26 per cent, thus being close to the global rates36,37,38,39,40,41,42,43,44,45,46,47,48,49,50. An eight-year audit from our institute using manual testing for ER and PR documented the highest rate of 56 per cent37. However, a six year analysis from 2009 to 2014 of 8270 patients revealed a hormone receptor positivity rate of up to 70 per cent (unpublished audit results) (Table). While anti-HER2neu drug herceptin arrived on horizon years ago for treating patients, laboratory guidelines in India have not been evolved. As there is no health insurance in place and patients pay for these tests in most institutes, there is a tendency for laboratories to economize. While the repertoire of antibodies available for ER/PR is limited, a bevy of antibodies are available in HER2neu testing. Due to high cost involved with the testing, most laboratories in India do non-FDA-approved/homebrew assays. As per the ASCO guidelines2, a laboratory is certified for HER2neu testing if the concordance rates are greater than 90 per cent for score 3+ and FISH amplified cases while only 1-5 per cent of 0/1+ are FISH amplified. However, a trend to play safe and give a high equivocal or score 2+ results to avoid false negatives or false positives has been observed, beating the purpose of IHC for HER2neu (unpublished observations). Furthermore, the primary cancer health care in breast cancer is often rendered by a physician or surgeon without any specific training in oncology. Hence, the patient often ends up with a specimen that is poorly fixed and not fit for evaluation, putting pressure on the referral cancer testing laboratories for ensuring test accuracy. Fig. 1A-D illustrates a stereotypical case encountered where a patient was treated as a triple-negative cancer based on reports from two centres when in fact, she had a HER2neu-amplified tumour. The main problem was that the poor primary fixation.
Table.
Rates for oestrogen receptor/progesterone receptor (ER/PR) and human epidermal growth factor receptor 2 (HER2neu) positivity reported from India
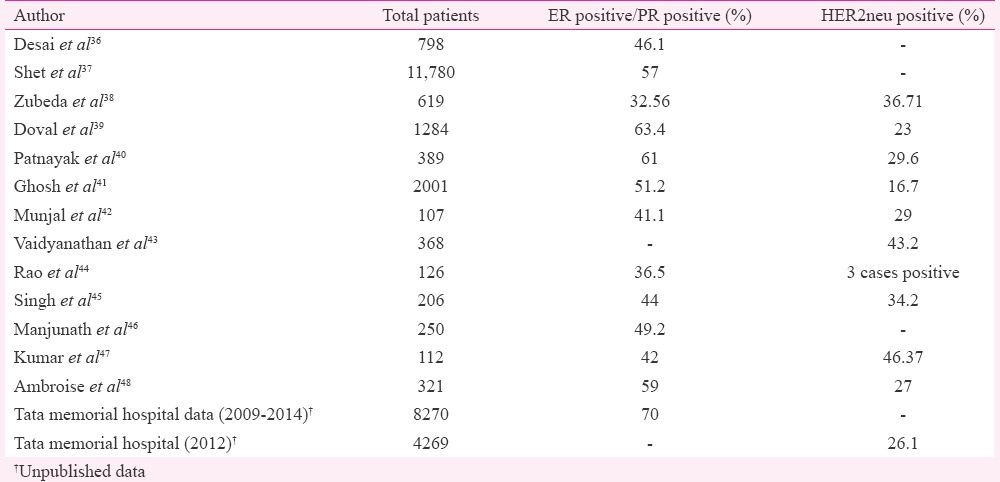
Fig. 1.
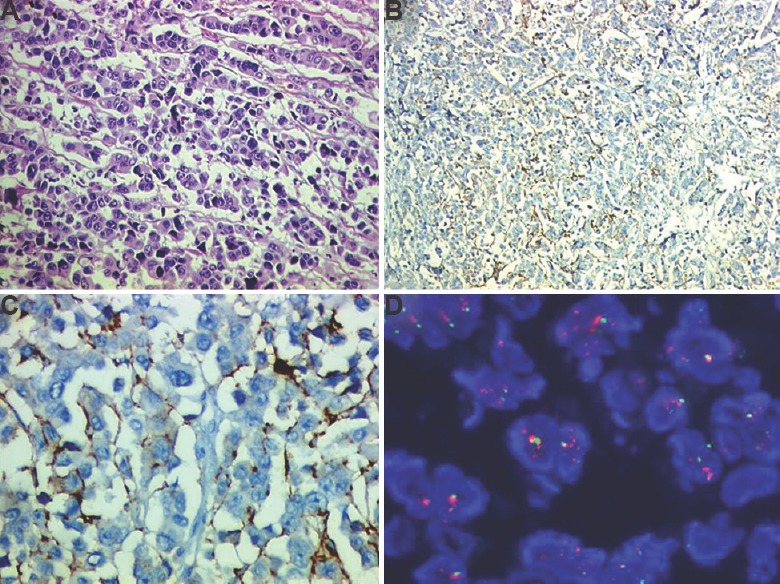
A patient affected by gap in biomarker testing (A) haematoxylin and eosin section confirmed that tumour was poorly fixed (H & E: 200×) (B) weak HER2neu staining due to poor fixation (immunoperoxidase: 200×), (C) higher power to indicate that HER2neu would be interpreted as score 1+/negative (immunoperoxidase: 400×), and (D) tumour as tested by fluorescent in situ hybridization found to be HER2neu amplified (Oil).
Issues plaguing hormone receptor and HER2neu testing
While ER/PR testing is influenced by fixation and antigen retrieval, for HER2neu, it is the use of validated antibody and interpretative error that result in discordant results. Issues that impact results of biomarkers in breast cancer can be broadly classified as into the following categories: (i) Pre-analytical factors; (ii) Variation in analytic methods chiefly platforms, antibodies and validation processes; and (iii) Post-analytical factors.
Pre-analytical factors
Improper fixation of tissue is the single most factor resulting in non-standardized results across low-resource countries where the site of specimen generation and testing laboratory are often differently located. Tissue specimens that are refrigerated or fixed in inadequate formalin are more likely to undergo autolysis and loose ER activity42,51,52.
In the UKNEQAS, a study of 25 tumours showed that a delay of up to 120 min in fixation resulted in reduction in ER immunopositivity25. Specimen are often refrigerated to delay loss of antigenicity; however, in a study of the 25 refrigerated samples, eight (32%), six (24%) and six (24%) cases showed reduction for ER, PR and HER2neu expression, respectively, in spite of refrigeration53. Khoury et al54 reported that overnight storage at 4°C resulted in loss of tissue antigenicity that was similar to leaving specimen without fixation for eight hours and recommended immediate specimen delivery rather than refrigeration. Specimens operated for breast cancer late in the week are more likely to be fixed later and hence more ER/PR negative than specimens obtained on other weekdays54. There is a strong contention for stopping PR testing in breast cancer to economize55. However, PR is a robust antigen less affected by fixation-related issues and often ensures hormonal therapy in a patient who is reported as ER negative falsely due to pre-analytical issues such as poor fixation. An eight-year audit of 11,000 odd cases at our institute revealed that improved fixation resulted in reduction in breast cancers that expressed only PR from 20 to three per cent because the improved fixation resulted in increased demonstration of ER and the category of tumours that expressed both ER/PR expanded37. ER-positive and PR-negative tumours are also less responsive to endocrine therapy (particularly tamoxifen) as opposed to ER-positive and PR-positive tumours helping prognostication56,57,58. PR negativity can also influence the therapeutic decision to offer adjuvant chemotherapy in addition to adjuvant endocrine therapy in selected patients59. Given the superior test results in core biopsy which are rapidly and better fixed, most developed countries perform testing for ER/PR and HER2neu on core biopsy60,61. However, core biopsies for primary diagnosis of breast cancer are not possible at all the places in resource-poor countries.
Analytical variables
The most common causes for discrepancy in analytical methods are non-validated antibodies, poorly calibrated ready-to-use products, insufficiently calibrated antibody dilutions (20%), insufficient or erroneous epitope retrieval (27%), less sensitive visualization systems (19%) and stainer platform-dependant protocol issues29.
The main advantage of automation in IHC is better-standardized retrieval and staining protocols. However, it is a dual-edged sword, and besides increasing costs, it separates the staff from the staining process increasing the likelihood of insufficient knowledge to handle troubleshooting issues62. As compared to manual staining automated immunostaining results in increased specificity, increased positive predictive value and increased efficiency of HER2neu test results63. Antigen retrieval with pressure cookers (besides fixation) was a major factor improving ER testing by manual staining in our laboratory37. Tissues fixed for less than six hours and with lesser antigen retrieval time (<25 min) had poor staining scores in one study52. How to select the best antibody for a specific antigen is complex but is aided by comparisons with a ‘gold standard’ and use of EQA data available from the UKNEQAS website (http://www.ukneqasiccish.org).
Several studies have reported their experience with different clones and companies for ER, PR and HER2neu antibodies. A study using standardized quantitative immunofluorescent ER assay demonstrated that SP1 clone for ER was at least eight per cent more sensitive and correlated better with patients’ outcome than 1D5 clone64. Significantly higher PR values were obtained when the tumours were analyzed by the Ventana 1E2 RTU kit compared to the PharmDX kit (clone PR 1294)65. The staining results with 4B5 antibody for HER2neu indicated that it had a more robust performance than CB11 clone and perfect correlation with FISH with excellent interlaboratory reproducibility66. A FISH and IHC comparison study at our institute with Immunotech antibody A revealed that 66.6 per cent of score 2+ cases showed amplification on FISH due to antibody-related issues50. Our observations (unpublished) with validated antibodies showed that only a quarter of our HER2neu equivocal cases were amplified. Results of validation and specificity of antibodies vary across the globe. For example, in UKNEQAS, 55-77 per cent of centres using 6F11 had satisfactory performance compared with only 35 per cent centres using 1D5, while another study documented that SP1 was a better antibody than 1D567,68. In an UKNEQAS study, while the laboratories using the DAKO Hercep test had the highest level of reproducibility in assay sensitivity and evaluation, the significant improvement in results by laboratories using other antibodies in the second assessment run suggested that stringent quality control and an ongoing quality assurance programme had the potential to improve the reliability of immunohistochemical assays for HER2neu, regardless of the brand of antibody used69. Though getting global uniformity in these analytical variables especially antibody clone seems difficult, getting systems in place and participation in an EQAS ensures minimal variability. Fig. 2A and B illustrates the same biopsy tested with two different methods showing different intensity of staining, demonstrating how variable analytic method can produce different results in the same sample.
Fig. 2.
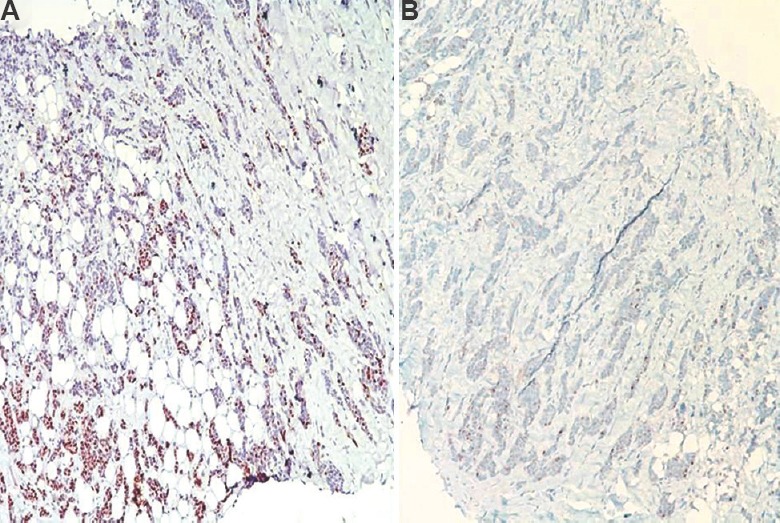
Same tumour stained using two protocols (A) manual staining gave a strong progesterone receptor staining (equal to Allred score 8/8), and (B) progesterone receptor testing in an automated stainer with same antibody gave weaker results (immunoperoxidase: 100×).
Post-analytical interpretative error
The interpretative error for hormone receptor reporting may be less as the cut-off value is small (1%)70. Interpretative error is the most important factor yielding variable accuracy rates for HER2neu testing. In one study, the overall concordance between observers for equivocal HER2neu results was low (55.8%) but for negative and positive results it was very high15. A unique trend of reporting was observed; pathologist with 100 per cent IHC and FISH concordance, usually had a tendency to play safe and reported a high number of equivocal cases while pathologists who reported clear cut results (positive or negative) had lesser concordance with FISH15. We observed that pathologist reporting both HER2neu IHC and FISH at our institute had better concordance with the FISH results71. Tumour heterogeneity is another cause for a false negative or positive report, and hence, it is always better to compare the tissue in core biopsy with excision specimen. PR is more heterogeneous than ER and may produce discordant results in core biopsy61. Carcinomas with HER2neu genetic heterogeneity can still have an overall negative HER2neu amplification status, despite still containing a significant number of tumour cells with HER2neu staining/score 3+ on IHC; hence, HER2neu heterogeneity should indicate need for FISH confirmation72,73.
Solutions to reduce the gap in ER/PR and HER2neu testing in India
Awareness and accountability
As the ASCO-CAP guidelines have highlighted, the good pathology practice starts in the operation theatre1,2,3. Surgeons should take additional responsibility of ensuring prompt transport of specimens or ensuring adequate fixation before these are dispatched to histopathology laboratory. Institutes should invest in training programmes for pathologist and technicians involved in an oncology service. There should be attempt at certification of laboratories before they sign out oncology and critical reports.
American Society of Clinical Oncology-College of American Pathologists (ASCO-CAP) guidelines
As per the ASCO-CAP 2007 HER2neu testing guidelines, samples where pre-analytical variables are unknown, had to be rejected74, but this would result in most samples in low-resource countries as inappropriate for testing. Mandatory participation in external proficiency testing programme with at least two testing events (mailings) per year is essential in ASCO-CAP guidelines1,2,3, making it an expensive mandate for most laboratories. These guidelines on the other hand, form a framework for use by all pathologists across the world to ensure uniformity. Most laboratories can also quote these guidelines to request for resources and infrastructure to achieve international standards. A CAP survey showed that following the ASCO-CAP guidelines for ER/PR testing, more laboratories are now including the exact specimen fixation time in their reports (37.9% in 2011 vs 27.2% in 2008)75.
Centralized testing
UKNEQAS documented that when histological material from different sources were collected centrally and subjected to a common protocol of antigen retrieval using automated immunohistochemical analysis and assessment, uniform results for ER/PR were obtained25. Furthermore, as HER2neu testing is considered a high-complexity test under the Clinical Laboratory Improvement Amendments of 1988, it must be internally validated to ensure accuracy and reproducibility before being offered by a laboratory76. Various countries have come up with the national guidelines to ensure that testing performed in a cost-effective manner and accurately and hence define the minimum number of test to be done, e.g. 100 in situ hybridization (ISH) tests (two per week) be performed in each centre per annum to qualify for reporting HER2neu ISH77. The fact remains that if pre-analytical conditions are below optimum, the use of a single laboratory and a standardized and automated staining method for ER/PR testing are not sufficient to reduce variability in ER/PR test results78.
Internal laboratory validation programme or audits
Regular and ongoing audit of ER/PR and HER2neu testing should be undertaken to monitor test variability. The standardized results could fail in some situations, e.g. batch-to-batch variations in antibody. Laboratories should audit their overall annual negative, equivocal and positive rate for HER2neu using a combination of IHC and ISH or compare test results with another laboratory. The CAP study revealed a tendency to follow ASCO-CAP guidelines, but there were several lacunae75. Of the laboratories comparing IHC HER2neu assays with an IHC test performed in another laboratory, only 56 per cent of laboratories used a recommended minimum of 25 cases75.
External quality assurance system (EQAS)
EQAS can provide guidance on how to achieve the best IHC standards and participation in such programmes helps laboratories detect problems not identified by internal quality control19,25,29. NordiQC reported that in the 14 runs of ER during 2003-2015, the proportion of sufficient stains increased from 45 per cent in the first run to about 70-90 per cent in the later runs29. In line with this observation, the pass rate for ‘old’ participants was consistently higher than for the new ones in the latest run 73 versus 51 per cent29. A web-based quality improvement training and a comparative study of accuracy of IHC tests of breast cancer biomarkers between a well-established laboratory in the United States and a field laboratory in Ibadan, Nigeria, demonstrated that this could be a useful and cost-effective tool for quality assurance of IHC and provide much-needed capacity building in resource-poor countries79.
In India while most of institutes have quality systems in place, it is driven by economics. Though the Indian Council of Medical Research (ICMR) has brought out a consensus document for management of breast cancer80, there is presently no forum that can bring these heterogeneous practices on one platform and form an India-based guidelines. The National Cancer Grid (NCG) funded by the Government of India through the Department of Atomic Energy was formed in August 2012 with the mandate of linking 69 cancer centres across India81. Forums like the NCG should initiate EQAS to establish guidelines that are implementable across the country. There should be a government mandate for all laboratories doing predictive marker testing to register with one of the forums. Once registered, the results of the participant laboratory should be monitored by a central laboratory annually and biannually. There is a need to divide the country into four zones (North, South, West and East) with centres of excellence in each zone covering all the testing laboratories in the region (Fig. 3).
Fig. 3.
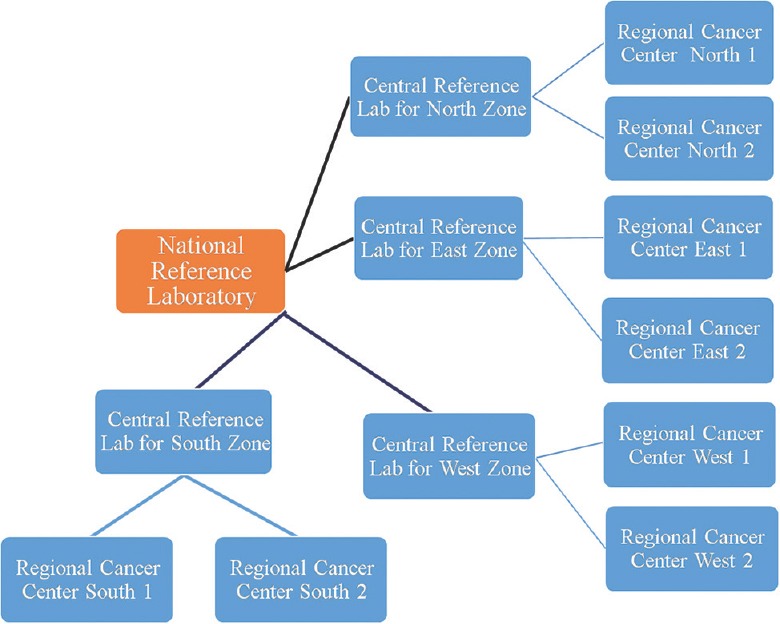
Schematic diagram of an organizational chart for future external quality assurance system programmes across the country.
Conclusion
There is a global initiative to recognize and rectify some of the loopholes in testing for ER/PR and HER2neu even in the low-resource countries. The variability in testing for these markers is rather wide in our country as compared to the other Asian nations and needs to be closed with an urgent national mandate by national bodies such as ICMR/NCG and by a team of professionals that encompass both government and health officials besides oncologists, surgeons and pathologists. Only then, one can ensure cost-effective and safe oncology care to the breast cancer patients.
Acknowledgment
Author acknowledges all the staff of Immunohistochemistry department especially Shri Mahadeo Patade, Dr Trupti Pai (Fellow, Molecular Pathology) and Dr Swapnil Rane (Assistant professor, Tata Memorial Hospital) for the data used.
Footnotes
Conflicts of Interest: The author is on Roche Advisory board for biomarker testing in India and member of the SPHERE programme.
References
- 1.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–95. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 3.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch Pathol Lab Med. 2014;138:241–56. doi: 10.5858/arpa.2013-0953-SA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ross JS, Symmans WF, Pusztai L, Hortobagyi GN. Standardizing slide-based assays in breast cancer: Hormone receptors, HER2, and sentinel lymph nodes. Clin Cancer Res. 2007;13:2831–5. doi: 10.1158/1078-0432.CCR-06-2522. [DOI] [PubMed] [Google Scholar]
- 5.Allred DC. Commentary: Hormone receptor testing in breast cancer: A distress signal from Canada. Oncologist. 2008;13:1134–6. doi: 10.1634/theoncologist.2008-0184. [DOI] [PubMed] [Google Scholar]
- 6.Hede K. Breast cancer testing scandal shines spotlight on black box of clinical laboratory testing. J Natl Cancer Inst. 2008;100:836–7. doi: 10.1093/jnci/djn200. 844. [DOI] [PubMed] [Google Scholar]
- 7.Carlson RW, Moench SJ, Hammond ME, Perez EA, Burstein HJ, Allred DC, et al. HER2 testing in breast cancer: NCCN task force report and recommendations. J Natl Compr Canc Netw. 2006;4(Suppl 3):S1–22. [PubMed] [Google Scholar]
- 8.Albanell J, Andreu X, Calasanz MJ, Concha A, Corominas JM, García-Caballero T, et al. Guidelines for HER2 testing in breast cancer: A national consensus of the Spanish society of pathology (SEAP) and the Spanish society of medical oncology (SEOM) Clin Transl Oncol. 2009;11:363–75. doi: 10.1007/s12094-009-0370-6. [DOI] [PubMed] [Google Scholar]
- 9.Haglund M, Chebil G, Johansson L. HER2 testing for breast cancer.Swedish laboratories can now offer a quality assured analysis of growth factor. Lakartidningen. 2005;102:740–3. [PubMed] [Google Scholar]
- 10.Francis GD, Dimech M, Giles L, Hopkins A. Frequency and reliability of oestrogen receptor, progesterone receptor and HER2 in breast carcinoma determined by immunohistochemistry in Australasia: Results of the RCPA quality assurance program. J Clin Pathol. 2007;60:1277–83. doi: 10.1136/jcp.2006.044701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Regitnig P, Reiner A, Dinges HP, Höfler G, Müller-Holzner E, Lax SF, et al. Quality assurance for detection of estrogen and progesterone receptors by immunohistochemistry in Austrian pathology laboratories. Virchows Arch. 2002;441:328–34. doi: 10.1007/s00428-002-0646-5. [DOI] [PubMed] [Google Scholar]
- 12.Grann VR, Troxel AB, Zojwalla NJ, Jacobson JS, Hershman D, Neugut AI, et al. Hormone receptor status and survival in a population-based cohort of patients with breast carcinoma. Cancer. 2005;103:2241–51. doi: 10.1002/cncr.21030. [DOI] [PubMed] [Google Scholar]
- 13.Calhoun BC, Collins LC. Predictive markers in breast cancer: An update on ER and HER2 testing and reporting. Semin Diagn Pathol. 2015;32:362–9. doi: 10.1053/j.semdp.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Atkinson R, Mollerup J, Laenkholm AV, Verardo M, Hawes D, Commins D, et al. Effects of the change in cutoff values for human epidermal growth factor receptor 2 status by immunohistochemistry and fluorescence in situ hybridization: A study comparing conventional brightfield microscopy, image analysis-assisted microscopy, and interobserver variation. Arch Pathol Lab Med. 2011;135:1010–6. doi: 10.5858/2010-0462-OAR. [DOI] [PubMed] [Google Scholar]
- 15.Green IF, Zynger DL. Institutional quality assurance for breast cancer HER2 immunohistochemical testing: Identification of outlier results and impact of simultaneous fluorescence in situ hybridization cotesting. Hum Pathol. 2015;46:1842–9. doi: 10.1016/j.humpath.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Viale G, Regan MM, Maiorano E, Mastropasqua MG, Dell’Orto P, Rasmussen BB, et al. Prognostic and predictive value of centrally reviewed expression of estrogen and progesterone receptors in a randomized trial comparing letrozole and tamoxifen adjuvant therapy for postmenopausal early breast cancer: BIG 1-98. J Clin Oncol. 2007;25:3846–52. doi: 10.1200/JCO.2007.11.9453. [DOI] [PubMed] [Google Scholar]
- 17.Rhodes A, Jasani B, Balaton AJ, Barnes DM, Anderson E, Bobrow LG, et al. Study of interlaboratory reliability and reproducibility of estrogen and progesterone receptor assays in Europe. Documentation of poor reliability and identification of insufficient microwave antigen retrieval time as a major contributory element of unreliable assays. Am J Clin Pathol. 2001;115:44–58. doi: 10.1309/H905-HYC1-6UQQ-981P. [DOI] [PubMed] [Google Scholar]
- 18.McCullough AE, Dell’orto P, Reinholz MM, Gelber RD, Dueck AC, Russo L, et al. Central pathology laboratory review of HER2 and ER in early breast cancer: An ALTTO trial [BIG 2-06/NCCTG N063D (Alliance)] ring study. Breast Cancer Res Treat. 2014;143:485–92. doi: 10.1007/s10549-013-2827-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pauletti G, Dandekar S, Rong H, Ramos L, Peng H, Seshadri R, et al. Assessment of methods for tissue-based detection of the HER-2/neu alteration in human breast cancer: A direct comparison of fluorescence in situ hybridization and immunohistochemistry. J Clin Oncol. 2000;18:3651–64. doi: 10.1200/JCO.2000.18.21.3651. [DOI] [PubMed] [Google Scholar]
- 20.Elkin EB, Weinstein MC, Winer EP, Kuntz KM, Schnitt SJ, Weeks JC, et al. HER-2 testing and trastuzumab therapy for metastatic breast cancer: A cost-effectiveness analysis. J Clin Oncol. 2004;22:854–63. doi: 10.1200/JCO.2004.04.158. [DOI] [PubMed] [Google Scholar]
- 21.Yaziji H, Gown AM. Accuracy and precision in HER2/neu testing in breast cancer: Are we there yet? Hum Pathol. 2004;35:143–6. doi: 10.1016/j.humpath.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Taucher S, Rudas M, Mader RM, Gnant M, Dubsky P, Bachleitner T, et al. Do we need HER-2/neu testing for all patients with primary breast carcinoma? Cancer. 2003;98:2547–53. doi: 10.1002/cncr.11828. [DOI] [PubMed] [Google Scholar]
- 23.Vera-Román JM, Rubio-Martínez LA. Comparative assays for the HER-2/neu oncogene status in breast cancer. Arch Pathol Lab Med. 2004;128:627–33. doi: 10.5858/2004-128-627-CAFTNO. [DOI] [PubMed] [Google Scholar]
- 24.Rhodes A, Jasani B, Barnes DM, Bobrow LG, Miller KD. Reliability of immunohistochemical demonstration of oestrogen receptors in routine practice: Interlaboratory variance in the sensitivity of detection and evaluation of scoring systems. J Clin Pathol. 2000;53:125–30. doi: 10.1136/jcp.53.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee H, Douglas-Jones AG, Morgan JM, Jasani B. The effect of fixation and processing on the sensitivity of oestrogen receptor assay by immunohistochemistry in breast carcinoma. J Clin Pathol. 2002;55:236–8. doi: 10.1136/jcp.55.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gregory DM, Parfrey PS. The breast cancer hormone receptor retesting controversy in Newfoundland and Labrador, Canada: Lessons for the health system. Healthc Manage Forum. 2010;23:114–8. doi: 10.1016/j.hcmf.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Sandoval C, Rahal R, Forte T, Klein-Geltink J, He D, Bryant H, et al. Indicator measures er/pr and her2 testing among women with invasive breast cancer. Curr Oncol. 2013;20:62–3. doi: 10.3747/co.20.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Makretsov N, Gilks CB, Alaghehbandan R, Garratt J, Quenneville L, Mercer J, et al. Development of an evidence-based approach to external quality assurance for breast cancer hormone receptor immunohistochemistry: Comparison of reference values. Arch Pathol Lab Med. 2011;135:874–81. doi: 10.5858/2010-0380-OAR1.1. [DOI] [PubMed] [Google Scholar]
- 29.Vyberg M, Nielsen S. Proficiency testing in immunohistochemistry - Experiences from nordic immunohistochemical quality control (NordiQC) Virchows Arch. 2016;468:19–29. doi: 10.1007/s00428-015-1829-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sayed S, Moloo Z, Wasike R, Bird P, Oigara R, Govender D, et al. Is breast cancer from sub Saharan Africa truly receptor poor? Prevalence of ER/PR/HER2 in breast cancer from Kenya. Breast. 2014;23:591–6. doi: 10.1016/j.breast.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Nichols HB, Trentham-Dietz A, Love RR, Hampton JM, Hoang Anh PT, Allred DC, et al. Differences in breast cancer risk factors by tumor marker subtypes among premenopausal Vietnamese and Chinese women. Cancer Epidemiol Biomarkers Prev. 2005;14:41–7. [PubMed] [Google Scholar]
- 32.Zhu X, Ying J, Wang F, Wang J, Yang H. Estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 status in invasive breast cancer: A 3,198 cases study at National Cancer Center, China. Breast Cancer Res Treat. 2014;147:551–5. doi: 10.1007/s10549-014-3136-y. [DOI] [PubMed] [Google Scholar]
- 33.Kim KM, Bilous M, Chu KM, Kim BS, Kim WH, Park YS, et al. Human epidermal growth factor receptor 2 testing in gastric cancer: Recommendations of an Asia-Pacific task force. Asia Pac J Clin Oncol. 2014;10:297–307. doi: 10.1111/ajco.12263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Cancer Registry Programme (ICMR) Consolidated report of population based cancer registries 2001-2004. Individual registry data. Bangalore, India: National Cancer Registry Programme (ICMR); 2001-2003. p. 161. [Google Scholar]
- 35.Murthy NS, Chaudhry K, Nadayil D, Agarwal UK, Saxena S. Changing trends in incidence of breast cancer: Indian scenario. Indian J Cancer. 2009;46:73–4. doi: 10.4103/0019-509x.48603. [DOI] [PubMed] [Google Scholar]
- 36.Desai SB, Moonim MT, Gill AK, Punia RS, Naresh KN, Chinoy RF, et al. Hormone receptor status of breast cancer in India: A study of 798 tumours. Breast. 2000;9:267–70. doi: 10.1054/brst.2000.0134. [DOI] [PubMed] [Google Scholar]
- 37.Shet T, Agrawal A, Nadkarni M, Palkar M, Havaldar R, Parmar V, et al. Hormone receptors over the last 8 years in a cancer referral center in India: What was and what is? Indian J Pathol Microbiol. 2009;52:171–4. doi: 10.4103/0377-4929.48909. [DOI] [PubMed] [Google Scholar]
- 38.Zubeda S, Kaipa PR, Shaik NA, Mohiuddin MK, Vaidya S, Pavani B, et al. Her-2/neu status: A neglected marker of prognostication and management of breast cancer patients in India. Asian Pac J Cancer Prev. 2013;14:2231–5. doi: 10.7314/apjcp.2013.14.4.2231. [DOI] [PubMed] [Google Scholar]
- 39.Doval DC, Sharma A, Sinha R, Kumar K, Dewan AK, Chaturvedi H, et al. Immunohistochemical profile of breast cancer patients at a tertiary care hospital in New Delhi, India. Asian Pac J Cancer Prev. 2015;16:4959–64. doi: 10.7314/apjcp.2015.16.12.4959. [DOI] [PubMed] [Google Scholar]
- 40.Patnayak R, Jena A, Rukmangadha N, Chowhan AK, Sambasivaiah K, Phaneendra BV, et al. Hormone receptor status (estrogen receptor, progesterone receptor), human epidermal growth factor-2 and p53 in South Indian breast cancer patients: A tertiary care center experience. Indian J Med Paediatr Oncol. 2015;36:117–22. doi: 10.4103/0971-5851.158844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghosh J, Gupta S, Desai S, Shet T, Radhakrishnan S, Suryavanshi P, et al. Estrogen, progesterone and HER2 receptor expression in breast tumors of patients, and their usage of HER2-targeted therapy, in a tertiary care centre in India. Indian J Cancer. 2011;48:391–6. doi: 10.4103/0019-509X.92245. [DOI] [PubMed] [Google Scholar]
- 42.Munjal K, Ambaye A, Evans MF, Mitchell J, Nandedkar S, Cooper K, et al. Immunohistochemical analysis of ER, PR, Her2 and CK5/6 in infiltrative breast carcinomas in Indian patients. Asian Pac J Cancer Prev. 2009;10:773–8. [PubMed] [Google Scholar]
- 43.Vaidyanathan K, Kumar P, Reddy CO, Deshmane V, Somasundaram K, Mukherjee G, et al. ErbB-2 expression and its association with other biological parameters of breast cancer among Indian women. Indian J Cancer. 2010;47:8–15. doi: 10.4103/0019-509X.58852. [DOI] [PubMed] [Google Scholar]
- 44.Rao C, Shetty J, Kishan Prasad HL. Morphological profile and receptor status in breast carcinoma: An institutional study. J Cancer Res Ther. 2013;9:44–9. doi: 10.4103/0973-1482.110358. [DOI] [PubMed] [Google Scholar]
- 45.Singh R, Gupta S, Pawar SB, Pawar RS, Gandham SV, Prabhudesai S, et al. Evaluation of ER, PR and HER-2 receptor expression in breast cancer patients presenting to a semi urban cancer centre in Western India. J Cancer Res Ther. 2014;10:26–8. doi: 10.4103/0973-1482.131348. [DOI] [PubMed] [Google Scholar]
- 46.Manjunath S, Prabhu JS, Kaluve R, Correa M, Sridhar TS. Estrogen receptor negative breast cancer in India: Do we really have higher burden of this subtype? Indian J Surg Oncol. 2011;2:122–5. doi: 10.1007/s13193-011-0072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar V, Tewari M, Singh U, Shukla HS. Significance of Her-2/neu protein over expression in Indian breast cancer patients. Indian J Surg. 2007;69:122–8. doi: 10.1007/s12262-007-0002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ambroise M, Ghosh M, Mallikarjuna VS, Kurian A. Immunohistochemical profile of breast cancer patients at a tertiary care hospital in South India. Asian Pac J Cancer Prev. 2011;12:625–9. [PubMed] [Google Scholar]
- 49.Navani S, Bhaduri AS. High incidence of oestrogen receptor negative progesterone receptor positive phenotype in Indian breast cancer: Fact or fiction? Indian J Pathol Microbiol. 2005;48:199–201. [PubMed] [Google Scholar]
- 50.Panjwani P, Epari S, Karpate A, Shirsat H, Rajsekharan P, Basak R, et al. Assessment of HER-2/neu status in breast cancer using fluorescence in situ hybridization & immunohistochemistry: Experience of a tertiary cancer referral centre in India. Indian J Med Res. 2010;132:287–94. [PubMed] [Google Scholar]
- 51.Arber DA. Effect of prolonged formalin fixation on the immunohistochemical reactivity of breast markers. Appl Immunohistochem Mol Morphol. 2002;10:183–6. doi: 10.1097/00129039-200206000-00015. [DOI] [PubMed] [Google Scholar]
- 52.Goldstein NS, Ferkowicz M, Odish E, Mani A, Hastah F. Minimum formalin fixation time for consistent estrogen receptor immunohistochemical staining of invasive breast carcinoma. Am J Clin Pathol. 2003;120:86–92. doi: 10.1309/QPHD-RB00-QXGM-UQ9N. [DOI] [PubMed] [Google Scholar]
- 53.Yildiz-Aktas IZ, Dabbs DJ, Bhargava R. The effect of cold ischemic time on the immunohistochemical evaluation of estrogen receptor, progesterone receptor, and HER2 expression in invasive breast carcinoma. Mod Pathol. 2012;25:1098–105. doi: 10.1038/modpathol.2012.59. [DOI] [PubMed] [Google Scholar]
- 54.Khoury T, Sait S, Hwang H, Chandrasekhar R, Wilding G, Tan D, et al. Delay to formalin fixation effect on breast biomarkers. Mod Pathol. 2009;22:1457–67. doi: 10.1038/modpathol.2009.117. [DOI] [PubMed] [Google Scholar]
- 55.Olivotto IA, Truong PT, Speers CH, Bernstein V, Allan SJ, Kelly SJ, et al. Time to stop progesterone receptor testing in breast cancer management. J Clin Oncol. 2004;22:1769–70. doi: 10.1200/JCO.2004.99.251. [DOI] [PubMed] [Google Scholar]
- 56.Ravdin PM, Green S, Dorr TM, McGuire WL, Fabian C, Pugh RP, et al. Prognostic significance of progesterone receptor levels in estrogen receptor-positive patients with metastatic breast cancer treated with tamoxifen: Results of a prospective Southwest Oncology Group study. J Clin Oncol. 1992;10:1284–91. doi: 10.1200/JCO.1992.10.8.1284. [DOI] [PubMed] [Google Scholar]
- 57.Bardou VJ, Arpino G, Elledge RM, Osborne CK, Clark GM. Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases. J Clin Oncol. 2003;21:1973–9. doi: 10.1200/JCO.2003.09.099. [DOI] [PubMed] [Google Scholar]
- 58.Fernö M, Stål O, Baldetorp B, Hatschek T, Källström AC, Malmström P, et al. Results of two or five years of adjuvant tamoxifen correlated to steroid receptor and S-phase levels. South Sweden breast cancer group, and South-East Sweden breast cancer group. Breast Cancer Res Treat. 2000;59:69–76. doi: 10.1023/a:1006332423620. [DOI] [PubMed] [Google Scholar]
- 59.Colozza M, Larsimont D, Piccart MJ. Progesterone receptor testing: Not the right time to be buried. J Clin Oncol. 2005;23:3867–8. doi: 10.1200/JCO.2005.05.167. [DOI] [PubMed] [Google Scholar]
- 60.Douglas-Jones AG, Collett N, Morgan JM, Jasani B. Comparison of core oestrogen receptor (ER) assay with excised tumour: Intratumoral distribution of ER in breast carcinoma. J Clin Pathol. 2001;54:951–5. doi: 10.1136/jcp.54.12.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hodi Z, Chakrabarti J, Lee AH, Ronan JE, Elston CW, Cheung KL, et al. The reliability of assessment of oestrogen receptor expression on needle core biopsy specimens of invasive carcinomas of the breast. J Clin Pathol. 2007;60:299–302. doi: 10.1136/jcp.2006.036665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Prichard JW. Overview of automated immunohistochemistry. Arch Pathol Lab Med. 2014;138:1578–82. doi: 10.5858/arpa.2014-0083-RA. [DOI] [PubMed] [Google Scholar]
- 63.Bánkfalvi A, Boecker W, Reiner A. Comparison of automated and manual determination of HER2 status in breast cancer for diagnostic use: A comparative methodological study using the Ventana BenchMark automated staining system and manual tests. Int J Oncol. 2004;25:929–35. [PubMed] [Google Scholar]
- 64.Welsh AW, Harigopal M, Wimberly H, Prasad M, Rimm DL. Quantitative analysis of estrogen receptor expression shows SP1 antibody is more sensitive than 1D5. Appl Immunohistochem Mol Morphol. 2013;21:139–47. doi: 10.1097/PAI.0b013e31825d73b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ekholm M, Grabau D, Bendahl PO, Bergh J, Elmberger G, Olsson H, et al. Highly reproducible results of breast cancer biomarkers when analysed in accordance with national guidelines - A Swedish survey with central re-assessment. Acta Oncol. 2015;54:1040–8. doi: 10.3109/0284186X.2015.1037012. [DOI] [PubMed] [Google Scholar]
- 66.Powell WC, Hicks DG, Prescott N, Tarr SM, Laniauskas S, Williams T, et al. A new rabbit monoclonal antibody (4B5) for the immunohistochemical (IHC) determination of the HER2 status in breast cancer: Comparison with CB11, fluorescence in situ hybridization (FISH), and interlaboratory reproducibility. Appl Immunohistochem Mol Morphol. 2007;15:94–102. doi: 10.1097/pai.0b013e31802ced25. [DOI] [PubMed] [Google Scholar]
- 67.Dowsett M. Estrogen receptor: Methodology matters. J Clin Oncol. 2006;24:5626–8. doi: 10.1200/JCO.2006.08.3485. [DOI] [PubMed] [Google Scholar]
- 68.Cheang MC, Treaba DO, Speers CH, Olivotto IA, Bajdik CD, Chia SK, et al. Immunohistochemical detection using the new rabbit monoclonal antibody SP1 of estrogen receptor in breast cancer is superior to mouse monoclonal antibody 1D5 in predicting survival. J Clin Oncol. 2006;24:5637–44. doi: 10.1200/JCO.2005.05.4155. [DOI] [PubMed] [Google Scholar]
- 69.Rhodes A, Jasani B, Anderson E, Dodson AR, Balaton AJ. Evaluation of HER-2/neu immunohistochemical assay sensitivity and scoring on formalin-fixed and paraffin-processed cell lines and breast tumors: A comparative study involving results from laboratories in 21 countries. Am J Clin Pathol. 2002;118:408–17. doi: 10.1309/97WN-W6UX-XJWT-02H2. [DOI] [PubMed] [Google Scholar]
- 70.Reisenbichler ES, Lester SC, Richardson AL, Dillon DA, Ly A, Brock JE, et al. Interobserver concordance in implementing the 2010 ASCO/CAP recommendations for reporting ER in breast carcinomas: A demonstration of the difficulties of consistently reporting low levels of ER expression by manual quantification. Am J Clin Pathol. 2013;140:487–94. doi: 10.1309/AJCP1RF9FUIZRDPI. [DOI] [PubMed] [Google Scholar]
- 71.Pai T, Shet T, Patil A, Desai S. Variability in HER2 testing: “Validation study in search of economic solution for India”. Modern Pathol. 2013;26:61. [Google Scholar]
- 72.Potts SJ, Krueger JS, Landis ND, Eberhard DA, Young GD, Schmechel SC, et al. Evaluating tumor heterogeneity in immunohistochemistry-stained breast cancer tissue. Lab Invest. 2012;92:1342–57. doi: 10.1038/labinvest.2012.91. [DOI] [PubMed] [Google Scholar]
- 73.Brunelli M, Manfrin E, Martignoni G, Miller K, Remo A, Reghellin D, et al. Genotypic intratumoral heterogeneity in breast carcinoma with HER2/neu amplification: Evaluation according to ASCO/CAP criteria. Am J Clin Pathol. 2009;131:678–82. doi: 10.1309/AJCP09VUTZWZXBMJ. [DOI] [PubMed] [Google Scholar]
- 74.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–45. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 75.Hardy LB, Fitzgibbons PL, Goldsmith JD, Eisen RN, Beasley MB, Souers RJ, et al. Immunohistochemistry validation procedures and practices: A College of American pathologists survey of 727 laboratories. Arch Pathol Lab Med. 2013;137:19–25. doi: 10.5858/arpa.2011-0676-CP. [DOI] [PubMed] [Google Scholar]
- 76.Middleton LP, Price KM, Puig P, Heydon LJ, Tarco E, Sneige N, et al. Implementation of American Society of Clinical Oncology/College of American Pathologists HER2 guideline recommendations in a tertiary care facility increases HER2 immunohistochemistry and fluorescence in situ hybridization concordance and decreases the number of inconclusive cases. Arch Pathol Lab Med. 2009;133:775–80. doi: 10.5858/133.5.775. [DOI] [PubMed] [Google Scholar]
- 77.Rakha EA, Pinder SE, Bartlett JM, Ibrahim M, Starczynski J, Carder PJ, et al. Updated UK recommendations for HER2 assessment in breast cancer. J Clin Pathol. 2015;68:93–9. doi: 10.1136/jclinpath-2014-202571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nkoy FL, Hammond ME, Rees W, Belnap T, Rowley B, Catmull S, et al. Variable specimen handling affects hormone receptor test results in women with breast cancer: A large multihospital retrospective study. Arch Pathol Lab Med. 2010;134:606–12. doi: 10.5858/134.4.606. [DOI] [PubMed] [Google Scholar]
- 79.Oluwasola AO, Malaka D, Khramtsov AI, Ikpatt OF, Odetunde A, Adeyanju OO, et al. Use of web-based training for quality improvement between a field immunohistochemistry laboratory in Nigeria and its United States-based partner institution. Ann Diagn Pathol. 2013;17:526–30. doi: 10.1016/j.anndiagpath.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 80.Indian Council of Medical Research. Consensus document for management of breast cancer. Prepared as an outcome of ICMR Subcommittee on Breast Cancer. New Delhi: ICMR; 2016. [Google Scholar]
- 81.Pramesh CS, Badwe RA, Sinha RK. The national cancer grid of India. Indian J Med Paediatr Oncol. 2014;35:226–7. doi: 10.4103/0971-5851.142040. [DOI] [PMC free article] [PubMed] [Google Scholar]


