Fig. 7.
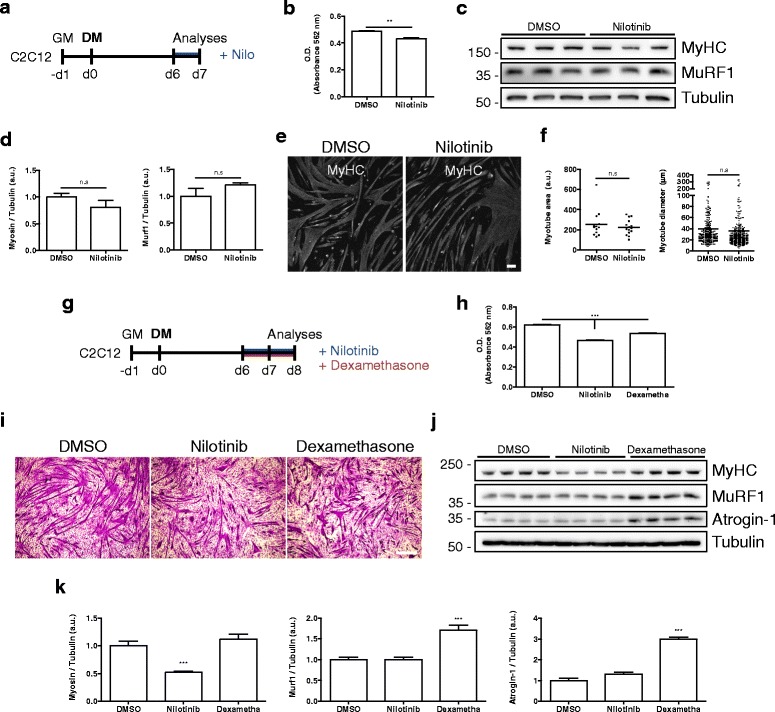
Nilotinib effects on differentiated myotubes. a Outline of the 24 h Nilotinib (Nilo) treatment protocol on day 6 C2C12-differentiated myotubes. b Total protein content was determined using BCA assay and O.D. obtained in control of Nilotinib-treated cells. n = 3; ***P < 0.001; DMSO vs Nilotinib; with two-tailed Student’s t test. c Western blot analysis of three representative experiments, evaluating the total amount of MyHC and MuRF1. Tubulin was used as the loading control. d Quantification of three independent experiments to evaluate MyHC and Murf1 expression. The values correspond to the mean ± SEM. n.s non-significant; n = 3; with two-tailed Student’s t test. e Immunofluorescence analyses showing the expression of MyHC (gray) in differentiated myotubes at day 7. Scale bar: 100 μm. f Quantification of three representative experiments, evaluating myotube area (left graph) and diameter (right graph). The values correspond to the mean ± SEM. n.s non-significant; n = 3; DMSO vs Nilotinib; with two-tailed Student’s t test. g Outline of 48 h Nilotinib and dexamethasone treatment protocol on day 6 C2C12-differentiated myotubes. Both compounds were added at 5-μM final concentration. h Total protein content was determined using BCA assay and O.D. obtained in control Nilotinib- or dexamethasone-treated cells. n = 3; ***P < 0.001; DMSO vs Nilotinib and DMSO vs dexamethasone (Dexametha); One-way ANOVA with Bonferroni post-test. i Representative images of crystal violet staining on day 8 myotubes. Scale bar: 500 μm. j, c Western blot analysis of four representative experiments, evaluating the total amount of MyHC, MuRF1, and Atrogin-1. Tubulin was used as the loading control. k Quantification of four independent experiments to evaluate MyHC, Murf1, and Atrogin-1 expression. Values correspond to the mean ± SEM. ***P < 0.001; n = 4. One-way ANOVA with Bonferroni post-test
