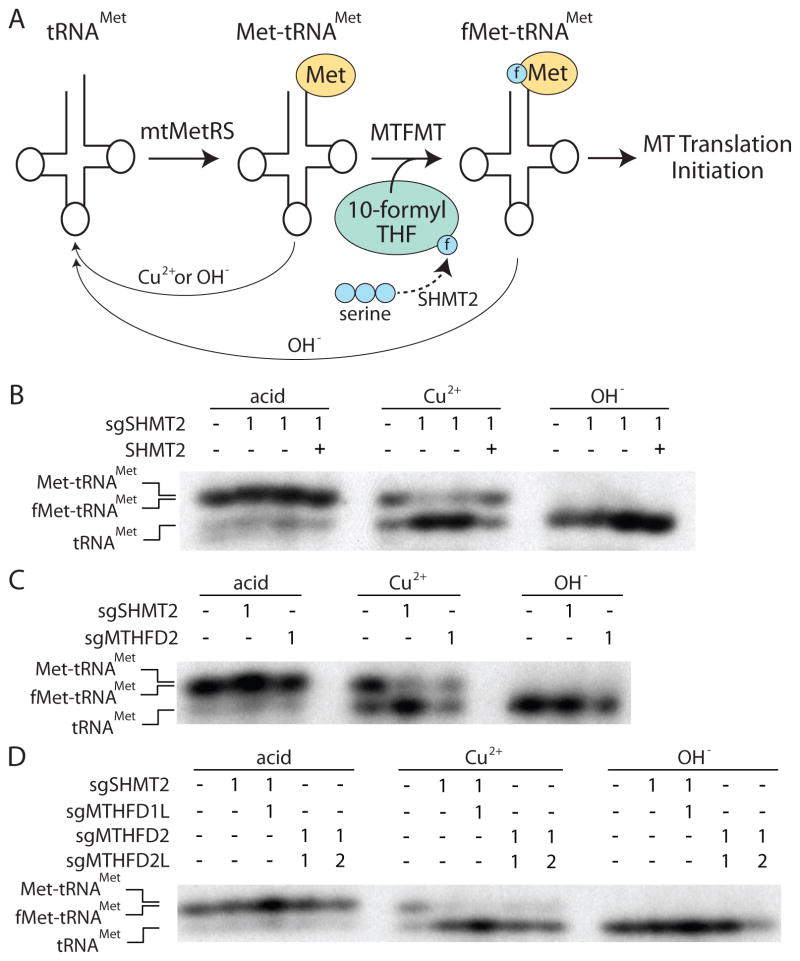
Figure 5. SHMT2-null cells are unable to maintain formylmethionyl-tRNA pools in mitochondria.
A, Schematic overview of mitochondrial tRNAMet maturation and sensitivity to Cu2+ or OH− treatment. Uncharged tRNAMet is charged by the mitochondrial methionyl tRNA synthetase (mtMetRS) to form Met-tRNAMet. This species is further modified by MTFMT using a formyl group (blue circle marked with an “f”), derived from serine via the SHMT2 reaction, forming fMet-tRNAMet. The fMet-tRNAMet is then used to initiate translation in the mitochondria. Upon treatment with Cu2+ or OH−, Met-tRNAMet is hydrolyzed to tRNAMet, whereas fMet-tRNAMet is relatively resistant to Cu2+ treatment. B–D, Detection of mitochondrial tRNAMet species by northern blot using a probe specific to the mitochondrial tRNAMet and RNA isolated from Jurkat cell clones expressing an sgRNA targeting SHMT2 or additionally re-expressing SHMT2, or clones expressing an sgRNA targeting MTHFD1L, MTHFD2, or MTHFD2L. Met and fMet modification of the tRNA alters its mobility as indicated on the left. RNA was isolated under acidic conditions to preserve fMet or Met charged species (left) or treated to selectively hydrolyze Met (Cu2+, middle) or non-selectively hydrolyze both fMet and Met (OH−, right).