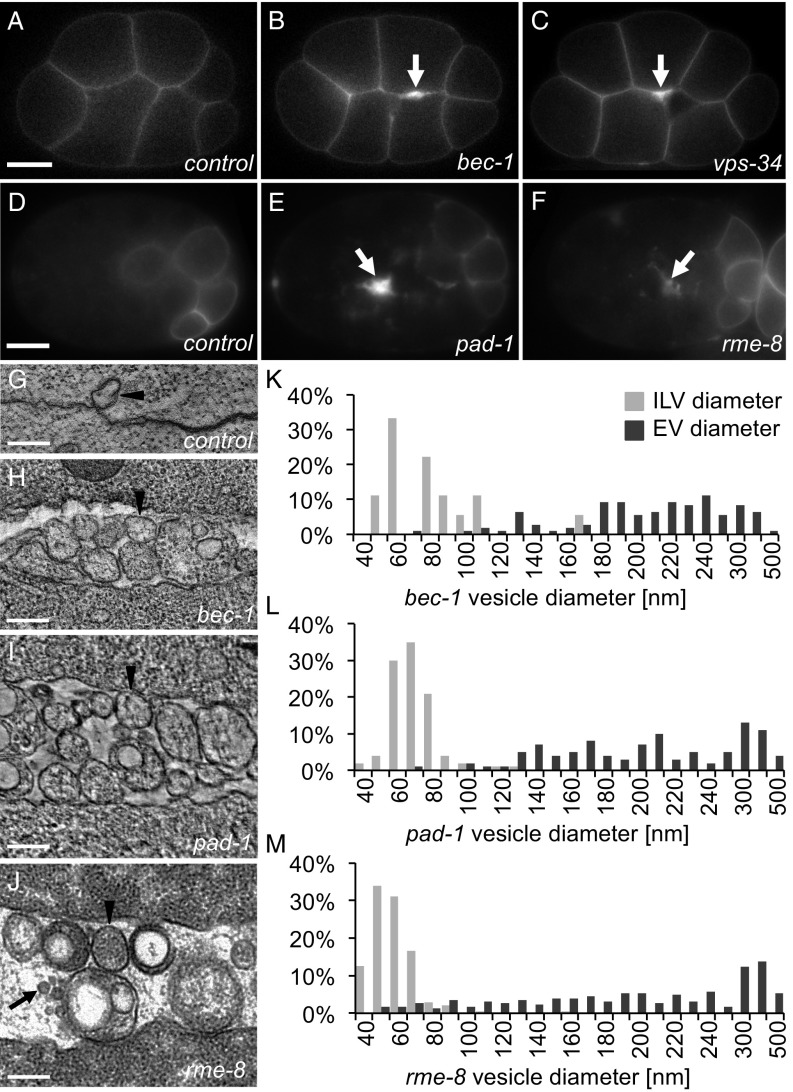
Fig. 1.
Retrograde trafficking proteins inhibit microvesicle release. (A) PHPLC1∂1::mCh localizes primarily to the plasma membrane in control embryos at the eight-cell stage. (B and C) PH reporters localize to thickened membranes (arrow) in bec-1 (PHPLC1∂1::mCh) and vps-34 (PHPLC1∂1::GFP) maternal zygotic mutants. (D) In a 26-cell control embryo, GFP::ZF1::PHPLC1∂1 is degraded in most somatic cells, only persisting on the plasma membrane in a few posterior cells. (E and F) EVs labeled with GFP::ZF1::PHPLC1∂1 (arrow) accumulate between cells in pad-1 and rme-8 RNAi-treated embryos. (G) EVs are infrequently observed between wild-type N2 cells in a two-cell embryo tomogram. (H–J) Released EVs accumulate between cells in a tomogram from a three-cell bec-1 maternal-zygotic mutant, a three-cell embryo treated with pad-1 RNAi, and a 24-cell rme-8 RNAi embryo. Arrowheads point to microvesicle-sized EVs; arrows point to exosome-sized EVs. (K–M) Histograms of EV and intraluminal vesicle (ILV) diameters measured from TEM images of bec-1 maternal-zygotic mutant embryos and pad-1 RNAi embryos demonstrate that the majority of EVs are microvesicles, because they are larger than ILVs. In rme-8 RNAi, EVs could be both exosomes and microvesicles, because they are the same size as or larger than ILVs. [Scale bars: A, 10 µm (also applies to B and C); D, 10 µm (also applies to E and F); G–J, 200 nm.]