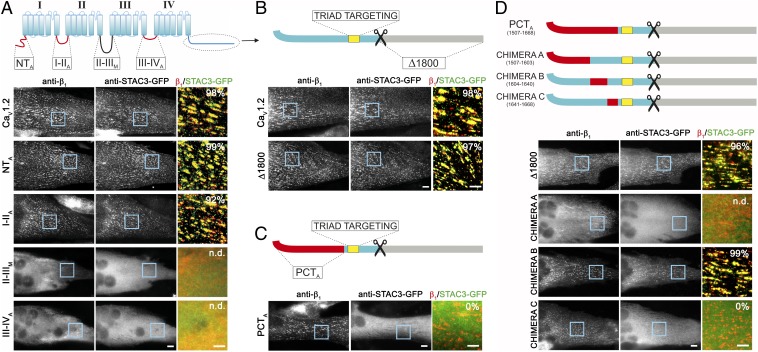
Fig. 1.
The PCT of CaV1.2 is crucial for the interaction with STAC3. (A–D, Upper) Cartoons showing the cytosolic domains of CaV1.2 (blue) substituted with the corresponding regions of CaV2.1 (N terminus, I-II loop, III-IV loop, PCT; red) or of the Musca channel (II-III loop; black). The CaV1.2 triad targeting sequence (yellow) is maintained in all chimeras. (Lower, Right) Representative immunofluorescence images of dysgenic myotubes expressing the indicated chimeric CaV1.2 channel and STAC3-GFP. (Scale bars, 10 μm.) (Lower, Right) Color overlay of CaVβ1a (red) and STAC3-GFP staining (green); 4× magnification of regions indicated by blue rectangle. (Scale bars, 5 μm.) The numbers indicate the percentage of myotubes in which STAC3-GFP was coclustered with the channel chimera (n = 3, n = 90). (A) STAC3-GFP showed triad targeting in the majority of the dysgenic myotubes expressing CaV1.2, CaV1.2-NTA or CaV1.2-I-IIA. CaV1.2-II-IIIM, and CaV1.2-III-IVA failed to localize in the triads of the myotubes, precluding analysis of STAC3-GFP association (n.d.). STAC3-GFP colocalized also with CaV1.2-Δ1800 (B), but not in myotubes expressing CaV1.2-PCTA (C), indicating that the PCT of CaV1.2 is crucial for the interaction with STAC proteins. (D) C-terminal chimera A was not targeted to the triads, preventing analysis of association of STAC3-GFP. Chimera B was targeted to the triads and STAC3-GFP colocalized in clusters, similarly to the control CaV1.2-Δ1800. Chimera C was targeted to the triads; however, it failed to recruit STAC3-GFP to the channel complex, indicating that amino acids 1,641–1,668 are crucial for the STAC interaction.