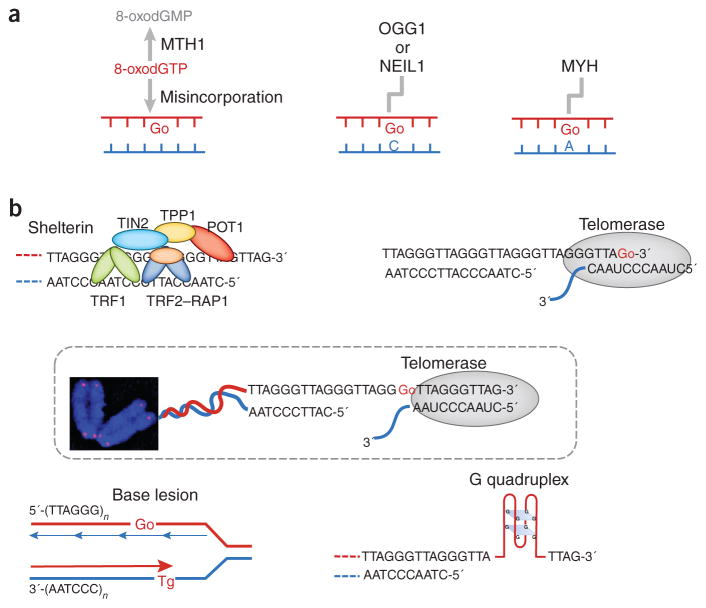
Figure 1.
Origin and consequences of oxidative DNA base damage within telomeres. (a) 8-oxoG (Go) in telomeres can arise from misincorporation of 8-oxodGTP or from in situ oxidation of a guanine DNA base. Proteins either hydrolyze the oxidized nucleotide pool or repair the oxidized bases within the DNA. (b) Telomere secondary structures, the telomeric proteins and oxidative DNA lesions influence elongation by telomerase. Top left, telomeric proteins negatively regulate telomerase-dependent elongation through the protein counting model (described in the main text). Oxidized bases compromise the binding of telomeric proteins to telomeres, thereby disrupting the counting mechanism. Top right, telomerase can utilize 8-oxoGTP during substrate extension, but 8-oxoGTP misincorporation terminates telomere elongation. Middle, telomerase uses its integral telomerase RNA as a template (with the sequence CAAUCCCAAUC) and adds the 6-nt repeating sequence 5′-TTAGGG-3′ to the 3′ overhang. Preexisting terminal 8-oxoG does not impair telomerase loading. Lower left, oxidized bases such as thymine glycol (Tg) may cause premature termination of telomere replication, thus leading to telomere shortening. Lower right, 8-oxoG ‘irons out’ G quadruplexes, thereby enhancing substrate accessibility and telomerase loading onto telomeres.