Fig. 1.
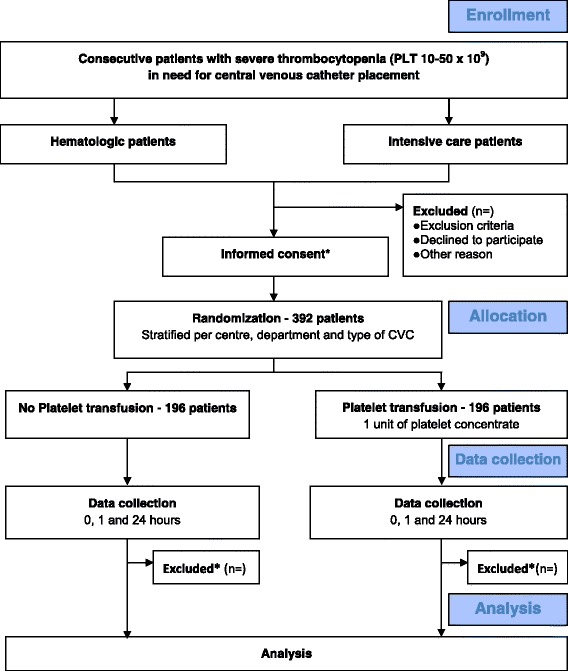
PACER trial (Consolidated Standards of Reporting Trials (CONSORT)) Diagram. CONSORT diagram of PACER. For haematologic patients, informed consent is required prior to randomisation. For the intensive care patients, randomisation takes place via deferred consent. If consent is not obtained, data are excluded
