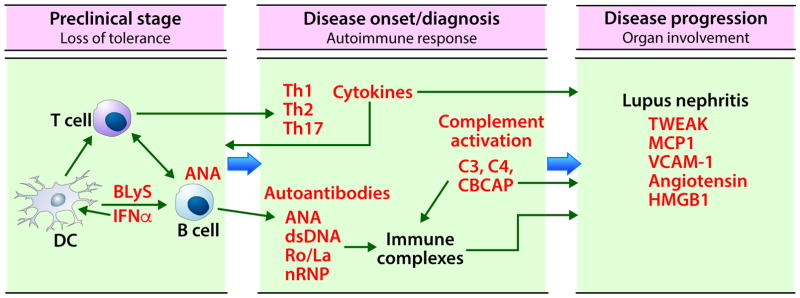
Figure 1. Biomarkers in SLE.
The initial stage in SLE pathogenesis is loss of tolerance to self-antigens. Genetic predisposition and environmental factors such as viral infections can lead to anti-nuclear antibody generation by epitope spreading. Disease onset or diagnosis occurs following the amplification of autoimmune response through the interactions between innate and adaptive immune cells (dendritic cells, T cells, and B cells), differentiation of T cells into Th1, Th2, Th17 subsets, and maturation and class switching of B cells to secrete autoantibodies. These autoantibodies form immune complexes, fix complement, and activate both classical and non-classical complement. This results in decreased complement C4 and C3, and an increase in cell bound C4d (CBCAP). The increased levels of cytokines, autoantibodies, and CBCAP, and decreased levels of C3 and C4 in circulation are the most useful biomarkers at this stage. Passive deposition of immune complexes or in situ immune complex formation in end organs such as kidneys, together with complement activation and high levels of secreted pro-inflammatory cytokines causes further organ damage. Urinary biomarkers are a convenient and effective way to monitor renal disease progression in SLE. DC; dendritic cells, BLyS: B lymphocyte stimulator, IFN: interferon, Th: T helper, ANA: antinuclear antibodies, dsDNA; anti double stranded DNA antibodies, nRNP; nuclear ribonuclear protein, TWEAK: TNF like weak inducer of apoptosis, MCP1: monocyte chemoattractant protein 1, VCAM-1: vascular adhesion molecule 1, HMGB1: high mobility group box 1 protein.