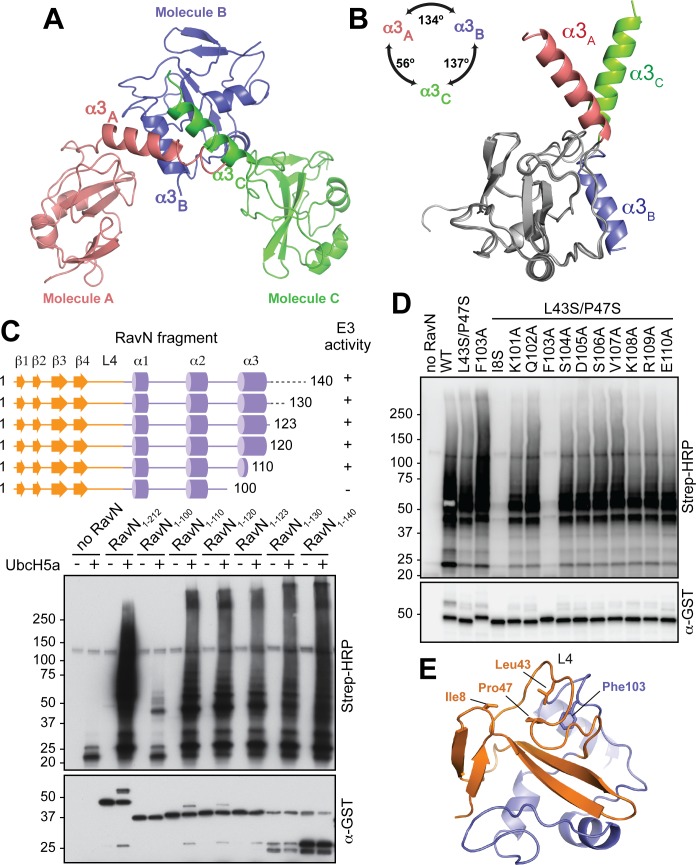
Fig 5. The flexible α3 helix is not required for the E3 ligase activity of RavN.
(A) Each asymmetric unit within the protein crystal contained a RavN1-123 molecule (shown as red, slate and green ribbon diagrams) that differed with respect to the orientation of their helix α3 (α3A, α3B, and α3C). (B) Superimposition of the three conformations of RavN1-123 found in the asymmetric unit. Structures are presented as ribbon diagrams, with the overlaid part colored in gray and the variable α3 helix colored in red, slate and green. (C) Top: Schematic representation of RavN fragments examined in the in vitro reconstitution assay (bottom) using UbcH5a as E2. Poly-ubiquitylated RavN was detected with HRP-conjugated streptavidin, and the total amount of GST-RavN present in each reaction was confirmed by immunoblot using anti-GST antibody. (D) Phe103 is important for E3 ligase activity of RavN. An in vitro reconstitution assay was used to determine the effect of the indicated amino acid substitutions within region 101–110 on the E3 ligase activity of RavNL43S/P47S. UbcH5a was added as E2 enzyme. The formation of poly-ubiquitin species was detected using HRP-conjugated streptavidin. The total amount of GST-RavN in each lane was determined by immunoblot using anti-GST antibody. (E) The position of Phe103 relative to the three E2 binding interface residues Ile8, Leu43, and Pro47 in RavN is shown. Color scheme as in Fig 3A.