Abstract
Aim
The aim of the study was to investigate the level of cytokines in cultures of cow’s milk protein- stimulated peripheral blood mononuclear cells of patients with cow’s milk protein allergy.
Material and Methods
Eleven children with cow’s milk protein allergy and 11 non-allergic controls were studied. Their peripheral blood mononuclear cells were cultured alone and in the presence of cow’s milk α-lactalbumin; β-lactoglobulin; αS 1, αS 2, β, and κ-casein fraction mixtures; and a cow’s protein mixture from whole milk. Production of cytokines, tumor necrosis factor-α, interleukin-10, and interleukin-12 were determined in culture supernatants.
Results
In cow’s milk protein-stimulated peripheral blood mononuclear cell cultures of children with cow’s milk protein allergy, tumor necrosis factor-α, interleukin-10, and interleukin-12 production was significantly higher than in non-allergic controls (p<0.05). No difference in cytokine production was found between cultures obtained from unstimulated peripheral blood mononuclear cell cultures of both cow’s milk protein allergy and non-allergic controls.
Conclusions
The findings of this preliminary study align with data from the literature suggesting that the investigation of tumor necrosis factor-α, interleukin-10, and interleukin-12 in cow’s milk protein-stimulated peripheral blood mononuclear cell cultures of children may be taken in further consideration to explore whether they might have a predictive role for cow’s milk protein allergy. Further studies are therefore needed to extensively investigate this issue.
Keywords: Challenge, cow milk, cytokines, immunoglobulin, interleukin, tumor necrosis factor
Introduction
Cow’s milk protein allergy (CMPA) is an adverse immunologic response to cow’s milk proteins (1). In particular, four casein fractions (αS 1, αS 2, β, and κ-casein) and two whey proteins (α-lactalbumin and β-lactoglobulin) are considered the most important allergenic proteins contained in cow’s milk (2). Cow’s milk protein allergy is one of the most common forms of food allergy in infants aged below 24 months in economically advanced countries (3) and its prevalence in this age group is estimated as 2–7.5%, whereas 5 to 15% of infants show symptoms suggesting adverse reactions to cow’s milk components (4,5). Preventing the onset of inappropriate elimination diets in children with CMPA is important (4). In fact, the only effective treatment for CMPA is the total avoidance of cows’ milk, which has to be replaced by appropriate substitutes (6), including soy formulas or casein, extensively hydrolyzed whey formulas or other mammalian milks such as goat’s milk (7), which is frequently used although several studies raised concerns regarding its tolerability and safety, particularly in children (3, 8, 9).
The immune reaction to cow’s milk proteins is determined by immunoglobulin (Ig)-E or non-IgE–mediated type of response (10). Currently, the diagnosis relies both on IgE tests, such as the skin prick test, and measurement of serum-specific IgE antibody levels to cow’s milk (1). However, the double-blind, placebo-controlled food challenge remains the diagnostic gold standard (11).
A T-cell–mediated reaction is the important first step in the initiation of an immune response (12), also involving the secretion of the natural and adaptive immunity mediators and cytokines by peripheral blood mononuclear cells (PBMCs) (13, 14), and it is considered to play a major role in delayed gastrointestinal reactions to cow’s milk proteins (15, 16). It has been demonstrated that peripheral blood lymphocytes derived from food-allergic patients display a higher proliferative capacity in response to food allergens as compared with non-allergic individuals (17). Previous studies have been performed with PBMCs in which cytokine production was measured (18). In particular, it has been suggested that interleukin (IL)-12 might play an important role in inhibiting inappropriate IgE synthesis and allergic inflammation as a result of allergen exposure (19). Furthermore, more recent reports have suggested that quantitation of cytokines, including tumor necrosis factor-α (TNF-α) and IL-10, in culture supernatants of cow’s milk-stimulated PBMCs could be considered as a diagnostic or predictive test to identify cow’s milk allergy among patients with immediate and non-immediate adverse reactions (2, 20, 21), and also helpful to reduce the need for food allergen challenges in young children (22).
In this preliminary study, we investigated the levels of regulatory cytokines TNF-α, IL-10, and IL-12 in culture supernatants of cow’s milk protein-stimulated PBMCs of children with CMPA.
Material and Methods
The study was performed by the University Unit of Pediatrics and Pediatric Research Center of the University of Foggia, Italy. Eleven patients with CMPA (six males and five females; mean age: 10.7 months, range, 3–36 months) and 11 non-allergic control subjects (six males and five females; mean age: 10.3 months, range, 4–39 months) were consecutively included in the study. All with subjects CMPA had various clinical manifestations suggestive of CMPA, including gastrointestinal, skin, and respiratory symptoms. In these subjects, CMPA was diagnosed through positive double-blind, placebo-controlled food challenge test results. Patients with metabolic disorders, anatomic abnormalities, coeliac disease, and other enteropathies, pancreatic insufficiency, non-immunologic adverse reactions to food, allergic reactions to other food allergens or other substances, malignancy, infections, and sepsis were excluded.
Informed consent for inclusion in the study was obtained from the parents. The study followed the requirements of the International Conference on Harmonisation Good Clinical Practice, respected the principles outlined by the Declaration of Helsinki on the Ethical Principles for Medical Research Involving Human Subjects, and was approved by the Ethics Committee for Pediatric Studies of the Medical School of the University of Foggia Prot. no.: 2-2.06/2010.
Cytokine determination in cultures of cow’s milk protein-stimulated peripheral blood mononuclear cells
The production of the regulatory cytokines TNF-α, IL-10, and IL-12 has been studied in cow’s milk protein-stimulated PBMCs from patients with CMPA and controls on the basis of their recognized major role as key regulators of allergic diseases (23–25). Venous blood samples were obtained from children with CMPA before the diagnostic elimination diet and challenge test with milk, and from controls (26). Peripheral blood mononuclear cells were isolated from these heparinized blood samples (3–5 mL) using gradient centrifugation in Ficoll–Hypaque (Sigma) (27). The peripheral blood mononuclear cells were suspended in Roswell Park Memorial Institute (RPMI) 1640 culture medium supplemented with glutamine, sodium pyruvate, nonessential amino acids, HEPES buffer, penicillin/streptomycin, and 10% FCS (Sigma). Peripheral blood mononuclear cells (0.2 mL) from subjects with CMPA and controls were distributed at a concentration of 1.5x105 cells per well into flat-bottomed 96-well micro-titer plates and cultivated for 5 days at 37° in a CO2 incubator in the presence of the following cow’s milk allergenic proteins: α-lactalbumin; β-lactoglobulin; αS 1, αS 2, β, and κ-casein fraction mixtures; and a cow’s protein mixture from whole milk obtained from the PrIME research center of the Faculty of Agriculture of the University of Foggia (2).
Milk proteins were used for stimulation at the concentration of 100 μg/mL. All culture conditions were set up in triplicate. Plates were incubated for 5 days at 37°C. Phytohemagglutinin (10 μg/mL) was used as a positive control. Peripheral blood mononuclear cells from patients with CMPA and controls were cultivated in RPMI medium only as negative control.
At the end of the incubation period, culture supernatants were harvested and stored at −20°C until used in cytokine assays. Interleukin-10, IL-12, and TNF-α levels were determined using commercial enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions; the results are expressed in pg/mL as mean±standard deviation.
Statistical Analysis
Statistical analyses were performed using the Statistical Package for Social Sciences V17 (IBM SPSS Statistics, Amonk, Ny, ABD) program. The Mann-Whitney U test was used to compare values that did not show normal distribution.
Differences with a p value <0.05 were considered significant.
Results
Tumor Necrosis Factor-α, Interleukin-10 and Interleukin-12 concentrations (pg/mL) in all culture supernatants of PBMCs from patients with CMPA cultivated in the presence of cow’s α-lactalbumin, β-lactoglobulin, caseins, and a protein mixture from whole milk, were significantly higher than in cultures from control subjects (p<0.05) (Figure 1). No difference in cytokine production was found between cultures obtained from unstimulated PBMCs of either patients with CMPA or controls.
Figure 1. a–c.
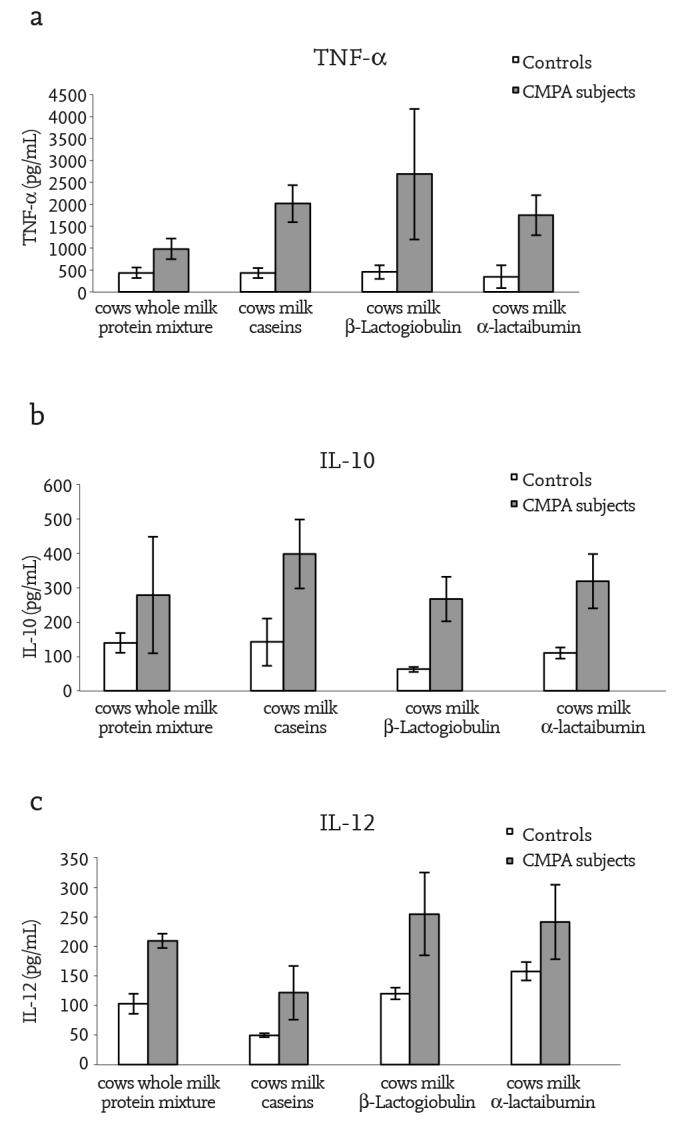
1α-lactalbumin; β-lactoglobulin; as 1, αS 2, β, and κ-casein fraction mixtures, and a protein mixture from whole milk. Tumor necrosis factor-α (TNF-α), interleukin-10 (IL-10) and interleukin-12 (IL-12) levels (pg/mL) in supernatants of peripheral blood mononuclear cell cultures of children with cow’s milk protein allergy and non-allergic controls stimulated with cow’s. Tumor necrosis factor-α concentration (p<0.05) (a), Interleukin-10 concentration (p<0.05) (b), Interleukin -12 concentration (p<0.05) (c)
Discussion
The gold standard in the diagnosis of food allergy with gastrointestinal symptoms is still based on the general principle of elimination and challenge with the offending antigen, and there is currently no widely accepted single, diagnostic laboratory test available to unveil the presence of an adverse immunologic response to cow’s milk proteins (3). T lymphocytes are a major source of cytokines (17), the hormonal messengers responsible for most of the biologic effects in the immune system. T-helper (Th) 1 cells are characterized by their ability to secrete interferon (IFN)-γ, IL-2, TNF-α, IL-12, and IL-15, whereas Th2 cells are characterized by IL-4, IL-5, IL-6, IL-10, and IL-13 secretion (28). Special attention has focused on TNF-α, and IL-10 and IL-12 as key regulators of allergic diseases (24, 26), where IL-12 and TNF-α promote Th1 responses and cellular immunity, whereas IL-10 suppresses Th1 activities and stimulates Th2 and humoral immune responses. A number of laboratory studies have been proposed to identify specific proteins responsible for allergic disorders related to CMPA (15, 16, 22, 29, 30). The possibility to use TNF-α, IL-10, and IL-12 in laboratory predictive tests for CMPA has been suggested in various studies, given the importance for children not to be exposed to the potentially dangerous reintroduction of the antigen (3, 22). In such a regard, some reports indicated that the measurement of cytokines in the culture supernatants of PBMCs may be useful for the diagnosis of CMPA, based on the evidence that high levels of cytokines, including TNF-α, IL-10, and IL-12 are released by the PBMCs of subjects with CMPA (22, 31).
Our data showed that, PBMCs from subjects with CMPA stimulated in culture by cow’s milk proteins, produced levels of TNF-α, IL-10, and IL-12 that were significantly higher than in PBMC’s from non-allergic control subjects tested with the same protein fractions. Previous studies separately investigated the activity of various cytokines, including TNF-α, IL-10, and IL-12, suggesting their possible role in unveiling CMPA (2, 20, 21). However, none of the available diagnostic tests are able to conclusively prove or disprove whether a child has CMPA (3). Allergen elimination diets and challenge procedures remain the gold standard for the diagnosis of CMPA in children (3, 11). The number of subjects with cow’s milk allergy and healthy controls evaluated in this study was limited. However, our findings align with data from the literature suggesting that the use of TNF-α, IL-10, and IL-12 in PBMC cultures could have a predictive role for CMPA (2, 20, 21). This finding is in contrast with other studies that used similar models, which found low levels of TNF-α and interferon (32). Therefore, further studies are needed to extensively investigate this issue. In addition, it is important to understand whether the changes in the production of these cytokines are specific enough to reveal CMPA in subjects with clinical symptoms suggestive of cow’s milk allergy.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Foggia University School of Medicine (No: 2-2.06/2010).
Informed Consent: Informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - M.P.M., M.D’A.; Design - M.P.M.; Supervision - M.P.M.; Funding - M.P.M.; Materials - I.G., A.C.; Data Collection and/or Processing - I.G., M.D’A.; Analysis and/or Interpretation - M.D’A.; Literature Review - M.P.M.; Writing - M.P.M; Critical Review - I.G.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Benhamou AH, Schäppi Tempia MG, Belli DC, Eigenmann PA. An overview of cow’s milk allergy in children. Swiss Med Wkly. 2009;139:300–7. doi: 10.4414/smw.2009.12258. [DOI] [PubMed] [Google Scholar]
- 2.Tiemessen MM, Van Ieperen-Van Dijk AG, Bruijnzeel-Koomen CAFM, Garssen J, Knol EF, Van Hoffen E. Cow’s milk–specific T-cell reactivity of children with and without persistent cow’s milk allergy: Key role for IL-10. J Allergy Clin Immunol. 2004;113:932–9. doi: 10.1016/j.jaci.2003.12.016. https://doi.org/10.1016/j.jaci.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 3.Vandenplas Y, Koletzko S, Isolauri E, et al. Guidelines for the diagnosis and management of cow’s milk protein allergy in infants. Arch Dis Child. 2007;92:902–8. doi: 10.1136/adc.2006.110999. https://doi.org/10.1136/adc.2006.110999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hill DJ, Hosking CS. Cow milk allergy in infancy and early childhood. Clin Exp Allergy. 1996;26:243–6. doi: 10.1111/j.1365-2222.1996.tb00086.x. https://doi.org/10.1111/j.1365-2222.1996.tb00086.x. [DOI] [PubMed] [Google Scholar]
- 5.Sicherer SH. Food Allergy. Lancet. 2002;360:701–10. doi: 10.1016/S0140-6736(02)09831-8. https://doi.org/10.1016/S0140-6736(02)09831-8. [DOI] [PubMed] [Google Scholar]
- 6.Giovannini M, Agostoni C, Fiocchi A, Bellu R, Trojan S, Riva E. Antigen-reduced infant formulas vs. human milk: growth and metabolic parameters in the first 6 months of life. J Am Coll Nutr. 1994;13:357–63. doi: 10.1080/07315724.1994.10718422. https://doi.org/10.1080/07315724.1994.10718422. [DOI] [PubMed] [Google Scholar]
- 7.Biggart T. Goat milk for the allergic child. Paediatr Today (UK) 1996;4:37–9. [Google Scholar]
- 8.Belloni-Businco B, Paganelli R, Lucenti P, Giampietro PG, Perborn H, Businco L. Allergenicity of goat’s milk in children with cow’s milk allergy. J Allergy Clin Immunol. 1999;103:1191–4. doi: 10.1016/s0091-6749(99)70198-3. https://doi.org/10.1016/S0091-6749(99)70198-3. [DOI] [PubMed] [Google Scholar]
- 9.Pessler F, Nejat M. Anaphylactic reaction to goat’s milk in a cow’s milk-allergic infant. Pediatr Allergy Immunol. 2004;15:183–5. doi: 10.1046/j.1399-3038.2003.00087.x. https://doi.org/10.1046/j.1399-3038.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- 10.Caffarelli C, Baldi F, Bendandi B, et al. Cow’s milk protein allergy. Ital J Pediatr. 2010;36:5. doi: 10.1186/1824-7288-36-5. https://doi.org/10.1186/1824-7288-36-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berni Canani R, Ruotolo S, Discepolo V, Troncone R. The diagnosis of food allergy in children. Curr Opin Pediatr. 2008;20:584–9. doi: 10.1097/MOP.0b013e32830c6f02. https://doi.org/10.1097/MOP.0b013e32830c6f02. [DOI] [PubMed] [Google Scholar]
- 12.Eigenmann PA. Mechanisms of food allergy. Pediatr Allergy Immunol. 2009;20:5–11. doi: 10.1111/j.1399-3038.2008.00847.x. https://doi.org/10.1111/j.1399-3038.2008.00847.x. [DOI] [PubMed] [Google Scholar]
- 13.Klein SC, Boer LH, de Wenger RA, de Gast GC, Bast EJEG. Release of cytokines and soluble cell surface molecules by PBMC after activation with the bispecific antibodies CD3xCD19. Scand J Immunol. 1997;46:452–8. doi: 10.1046/j.1365-3083.1997.d01-151.x. https://doi.org/10.1046/j.1365-3083.1997.d01-151.x [DOI] [PubMed] [Google Scholar]
- 14.Crucian B, Dunne P, Friedman H, Ragsdale R, Pross S, Widen R. Alterations in peripheral blood mononuclear cell cytokine production in response to phytoemagglutinin in multiple sclerosis patients. Clin Diagn Lab Immunol. 1995;2:766–9. doi: 10.1128/cdli.2.6.766-769.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill DJ, Ball G, Hosking CS. Clinical manifestations of cows’ milk allergy in childhood: associations with in-vitro cellular immune responses. Clin Allergy. 1998;18:469–79. doi: 10.1111/j.1365-2222.1988.tb02897.x. https://doi.org/10.1111/j.1365-2222.1988.tb02897.x. [DOI] [PubMed] [Google Scholar]
- 16.Suomalainen H, Soppi E, Isolauri E. Lymphocyte response to cow’s milk proteins in patients with cow’s milk allergy: relationship to antigen exposure. Pediatr Allergy Immunol. 1994;5:20–6. doi: 10.1111/j.1399-3038.1994.tb00214.x. https://doi.org/10.1111/j.1399-3038.1994.tb00214.x. [DOI] [PubMed] [Google Scholar]
- 17.Bohle B. T lymphocytes and food allergy. Mol Nutr Food Res. 2004;48:424–33. doi: 10.1002/mnfr.200400003. https://doi.org/10.1002/mnfr.200400003. [DOI] [PubMed] [Google Scholar]
- 18.Desjeux JF, Heyman M. Milk proteins, cytokines and intestinal epithelial functions in children. Acta Paediatr Jpn. 1994;36:592–6. doi: 10.1111/j.1442-200x.1994.tb03251.x. https://doi.org/10.1111/j.1442-200X.1994.tb03251.x. [DOI] [PubMed] [Google Scholar]
- 19.Chung F. Anti-inflammatory cytokines in asthma and allergy: interleukin-10, interleukin-12, interferon-γ. Mediators Inflamm. 2001;10:51–9. doi: 10.1080/09629350120054518. https://doi.org/10.1080/09629350120054518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heyman M, Desjeux JF. Cytokine-induced alteration of the epithelial barrier to food antigens in disease. Ann N Y Acad Sci. 2000;915:304–11. doi: 10.1111/j.1749-6632.2000.tb05258.x. https://doi.org/10.1111/j.1749-6632.2000.tb05258.x. [DOI] [PubMed] [Google Scholar]
- 21.Benlounes N, Candalh C, Matarazzo P, Dupont C, Heyman M. The time-course of milk antigen-induced TNF-alpha secretion differs according to the clinical symptoms in children with cow’s milk allergy. J Allergy Clin Immunol. 1999;104:863–9. doi: 10.1016/s0091-6749(99)70300-3. https://doi.org/10.1016/S0091-6749(99)70300-3. [DOI] [PubMed] [Google Scholar]
- 22.Motrich RD, Gottero C, Rezzonico C, Riera CM, Rivero V. Cow’s milk situmulated lymphocyte prolipheration and TNFalpha secretion in hypersensitivity to cow’s milk protein allergy. Clin Immunol. 2003;109:203–11. doi: 10.1016/s1521-6616(03)00182-7. https://doi.org/10.1016/S1521-6616(03)00182-7. [DOI] [PubMed] [Google Scholar]
- 23.Høst A, Koletzko B, Dreborg S, et al. Dietary products used in infants for treatment and prevention of food allergy. Arch Dis Child. 1999;81:80–4. doi: 10.1136/adc.81.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hagendorens MM, Didier G, Ebo DG, Bridts CH, De Clerck LS, Stevens WJ. Flow cytometrical determination of regulatory cytokines (IL-10, IL-12) and circulating dendritic cell cytokines in allergic asthmatic children. Cytokine. 2004;26:82–8. doi: 10.1016/j.cyto.2004.01.001. https://doi.org/10.1016/j.cyto.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Brightling C, Berry M, Amrani Y. Targeting TNF-alpha: a novel therapeutic approach for asthma. J Allergy Clin Immunol. 2008;121:5–10. doi: 10.1016/j.jaci.2007.10.028. https://doi.org/10.1016/j.jaci.2007.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krogulska A, Wasowska-Królikowska K, Polakowska E, Chrul S. Cytokine profile in children with asthma undergoing food challenges. J Investig Allergol Clin Immunol. 2009;19:43–8. [PubMed] [Google Scholar]
- 27.Kim A, Pettoello-Mantovani M, Goldstein H. Decreased susceptibility of peripheral blood mononuclear cells from individuals heterozygous for a mutant CCR5 allele to HIV infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19:145–9. doi: 10.1097/00042560-199810010-00007. https://doi.org/10.1097/00042560-199810010-00007. [DOI] [PubMed] [Google Scholar]
- 28.Mediaty A, Neuber K. Total and specific serum IgE decreases with age in patients with allergic rhinitis, asthma and insect allergy but not in patients with atopic dermatitis. Immun Ageing. 2005;2:9. doi: 10.1186/1742-4933-2-9. https://doi.org/10.1186/1742-4933-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dupont C, Heyman M. Food protein-induced enterocolitis syndrome: laboratory perspectives. J Pediatr Gastroenterol Nutr. 2000;30:S50–7. doi: 10.1097/00005176-200001001-00008. https://doi.org/10.1097/00005176-200001001-00008. [DOI] [PubMed] [Google Scholar]
- 30.Saarinen M, Suomalainen H, Savilahti E. Diagnostic value of skin-prick and patch tests and serum eosinophil cationic protein and cow’s milk-specific IgE in infants with cow’s milk allergy. Clin Exp Allergy. 2001;31:423–9. doi: 10.1046/j.1365-2222.2001.01015.x. https://doi.org/10.1046/j.1365-2222.2001.01015.x. [DOI] [PubMed] [Google Scholar]
- 31.Jyonouchi H, Geng L, Ruby A, Reddy C, Zimmerman-Bier B. Evaluation of an association between gastrointestinal symptoms and cytokine production against common dietary proteins in children with autism spectrum disorders. J Pediatr. 2005;146:605–10. doi: 10.1016/j.jpeds.2005.01.027. https://doi.org/10.1016/j.jpeds.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 32.Osterlund P, Järvinen KM, Laine S, Suomalainen H. Defective tumor necrosis factor- alpha production in infants with cow’s milk allergy. Pediatr Allergy Immunol. 1999;10:186–90. doi: 10.1034/j.1399-3038.1999.00034.x. https://doi.org/10.1034/j.1399-3038.1999.00034.x. [DOI] [PubMed] [Google Scholar]


