Figure 10.
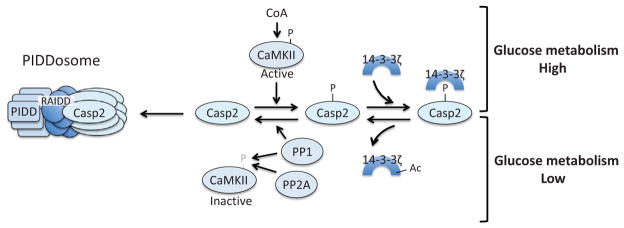
Caspase-2 is metabolically regulated. In a state of glucose abundance, CoA-activated CaMKII phosphorylates caspase-2, which creates a binding site for 14-3-3ζ and keeps caspase-2 inactive. In periods of low glycolytic flux, 14-3-3ζ is acetylated, which triggers release of this adaptor protein from caspase-2, allowing for PP1-mediated dephosphorylation of caspase-2. PP1 and PP2A also dephosphorylate and inactivate CaMKII. The dephosphorylated form of caspase-2 can then be recruited to the PIDDsome for activation.