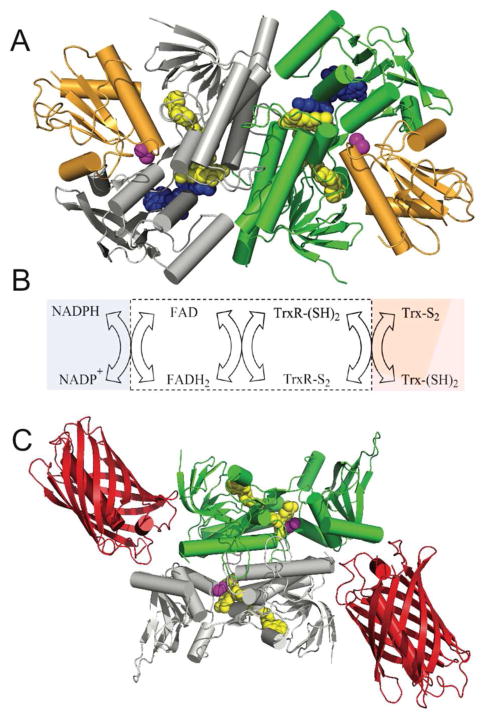
Figure 3.
E. coli thioredoxin reductase: structure, reactions and probe design. Panel A shows the three dimensional structure of E. coli thioredoxin reductase (PDB: 1F6M). The two subunits of this homodimer are depicted in green and grey and the bound thioredoxin (Trx1) is shown in orange. The space-filling representations of the flavin cofactor, a redox active disulfide, and the NADP+ analog, 3-aminopyridine adenine dinucleotide phosphate are in yellow, magenta, and blue respectively. Panel B shows the reductive and oxidative half-reactions without regard to protonation states of the reactants and products. The flavin and redox-active disulfide moieties of the reductase lie within the white box. Panel C is a schematic depiction of the sensor protein with the mCherry fluorescent protein (PDB: 2H5Q) fused to the C-terminus of thioredoxin reductase (PDB: 1CL0).