Table 2.
Catalytic hydroboration of aldehydes and ketones using 1tLi precatalyst in C6D6.
| Aldehyde/Ketone | t [h] | Yield as determined by 1H NMR [%][a] | |
|---|---|---|---|
| 1 |
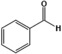
|
0.25 | >99 [>95][c] |
| 2 |
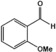
|
0.25 | 93 |
| 3 |
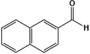
|
0.25 | 98 |
| 4 |
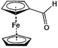
|
0.25 | >99 |
| 5 |
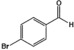
|
0.25 | >99 |
| 6 [b] |
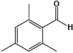
|
24 | >99 |
| 7 |
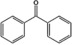
|
0.5 | 97 |
| 8 |
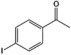
|
0.25 | >99 |
| 9 |
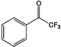
|
0.25 | >99 |
| 10 |
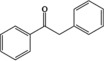
|
0.25 | >99 |
| 11 |
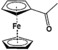
|
0.25 | 97 |
| 12 |
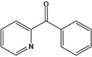
|
0.25 | >98 |
| 13 [b] |
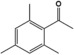
|
24 | 89 |
| 14 |
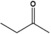
|
0.25 | 96 |
| 15 [b] |
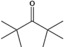
|
24 | 69 |
[a] Yield determined by formation of RR'CHOBpin relative to internal standard hexamethylcyclotrisiloxane. [b] Heated at 70 °C. [c] 1 % catalyst loading.
