ABSTRACT
Mycoplasma bovis-induced immune suppression is a major obstacle faced by the host for controlling infections. M. bovis impairment of antigen-specific T-cell responses is achieved through inhibiting the proliferation of peripheral blood mononuclear cells (PBMCs). This impairment may contribute to the persistence of M. bovis infection in various sites, including lungs, and its systemic spread to various organs such as joints, with the underlying mechanisms remaining elusive. Here, we elucidated the role of the immune-inhibitory receptor programmed death 1 (PD-1) and its ligand (PD-L1) in M. bovis infection. Flow cytometry (FCM) analyses revealed an upregulation of PD-L1 expression on tracheal and lung epithelial cell lines after M. bovis infection. In addition, we found increased PD-L1 expression on purified lung lavage macrophages following M. bovis infection by FCM and determined its localization by immunofluorescence analysis comparing infected and control lung tissue sections. Moreover, M. bovis infection increased the expression of the PD-1 receptor on total PBMCs and in gated CD4+ and CD8+ T-cell subpopulations. We demonstrated that M. bovis infection induced a significant decrease in CD4+ PD-1INT and CD8+ PD-1INT subsets with intermediate PD-1 expression, which functioned as progenitor pools giving rise to CD4+ PD-1HIGH and CD8+ PD-1HIGH subsets with high PD-1 expression levels. We blocked PD-1 receptors on PBMCs using anti-PD-1 antibody at the beginning of infection, leading to a significant restoration of the proliferation of PBMCs. Taken together, our data indicate a significant involvement of the PD-1/PD-L1 inhibitory pathway during M. bovis infection and its associated immune exhaustion, culminating in impaired host immune responses.
KEYWORDS: Mycoplasma bovis, cattle, PD-1, PD-L1, PBMC
INTRODUCTION
Mycoplasma-related infections in cattle are ranked as a top priority globally by the Office International des Epizooties (1). Cattle producers all over the world suffer significant economic losses due to morbidity and mortality associated with Mycoplasma bovis infections (2). M. bovis is a small pleomorphic bacterium with no cell wall, which affects both feedlot cattle (chronic pneumonia and polyarthritis syndrome [CPPS]) and dairy cattle (therapy-resistant mastitis) (3). This pathogen is able to evade the host immune system by the induction of substantial antigenic variation using its variable surface proteins (VSPs) (4). To date, limited success is achieved in the treatment or control of disease associated with M. bovis due to its virulence and persistent nature, restrictions on antimicrobial drug usage in food animals, the emergence of drug-resistant strains (5, 6), and vaccination attempts that confer limited protection (7–9).
We know that variable outcomes of infection are significantly dependent on the capacity of the host immune system to identify pathogens (10). Inhibition of immune responses by invading pathogens is considered one of the fundamental reasons for pathogens evading host immunity (11). Emerging vaccine trials have led to the exploration of various immunosuppressive strategies that contribute to limited host immune responses aimed at members of the CD28 receptor family such as the programmed death 1 (PD-1 or CD279) receptor and its ligands (12). The PD-1 receptor is broadly expressed on activated T cells, regulatory T cells, and other cells of a hematopoietic lineage (13). The PD-1 receptor recognizes PD ligand 1 (PD-L1 or CD274) and PD ligand 2 (PD-L2 or CD273), which are normally expressed on activated macrophages (Mϕ) and dendritic cells (12). Engagement of PD-1 with its ligands may lead to the exhaustion of the effector T-cell population, resulting in limited responses to infections or tumor cells (13, 14). It has been shown that this PD-1/PD-L1 inhibitory axis results in the significant attenuation of T-cell receptor signaling, decreased expression levels of autocrine and paracrine cytokines, a marked inhibition of proliferation, and reduced cytotoxic activity (15). PD-1 upregulation leads to the immune exhaustion of CD4+ and CD8+ T cells (16). Two different subset of T cells have been reported based on their levels of expression of the PD-1 molecule as intermediate-expression PD-1INT and high-expression PD-1HIGH T cells during chronic infections (17), where T cells can become progressively exhausted, such as during chronic lymphocytic choriomeningitis virus (LCMV) infection (18). Nevertheless, both PD-1INT and PD-1HIGH T cells are needed for controlling chronic infections, but the shift from a PD-1INT T-cell subset to a terminally differentiated PD-1HIGH T-cell subset led to poor outcomes (17). Previous studies suggest the importance of reinvigorating mainly the PD-1INT T-cell subset by targeted blocking in order to reduce viral infection and improve T-cell functions (19).
Various chronic lung infections in humans caused by both bacterial and viral pathogens have exhibited the involvement of the PD-1 receptor (20–22). Similar studies have recently investigated the PD-1 receptor-specific immunosuppressive role in various chronic infections in cattle (16). The PD-1/PD-L1 axis has been studied for bovine leukemia virus infection (23) and Mycoplasma bovis infection (24). Those studies suggest that the PD-1/PD-L1 interaction plays a crucial role in shutting down vital functions of antigen-specific T cells, leading to their exhaustion. In addition, it has been shown that the engagement of either PD-1 or PD-L1 with specific blocking antibodies allowed the restoration of T-cell functions, such as proliferation, cytokine production, and cytotoxic capacity, in both ex vivo and in vivo models (23, 25–27).
M. bovis is capable of invading erythrocytes and peripheral blood mononuclear cell (PBMC) subsets in vitro, causing the inhibition of their proliferation and delayed apoptosis (28, 29). M. bovis can also invade bovine tracheal epithelial (EBTr) and bovine lung epithelial (EBL) cells and survive in monocytes and bronchoalveolar lavage fluid macrophages (BAL-Mϕ) (6). Previous studies by our group showed that the inhibition of lymphocyte proliferation is a putative mechanism employed by M. bovis for immune evasion (29). Based on those observations, it was interesting to further elucidate the underlying mechanisms involved. The objective of this study is to characterize the expression of PD-1 and PD-L1 in uninfected healthy bovine cells and in cells following infections with M. bovis. We determined the expression levels of PD-L1 on EBTr cells, EBL cells, and BAL-Mϕ and of PD-1 on bovine PBMC subsets. Since M. bovis infection of PBMCs causes an inhibition of proliferation even in the presence of a potent mitogenic signal by concanavalin A (ConA) (28), we hypothesized that blocking the interaction of PD-1 with its ligand could help restore normal T-cell proliferation. A recent study showed an enhancement of interferon gamma production via PD-1/PD-L1 blockade in bovine mycoplasmosis (24), and many similar findings were reported previously for infections by several other pathogens such as Anaplasma marginale (30), Plasmodium berghei (31), Mycobacterium avium subsp. paratuberculosis (26), and bovine leukemia virus (23, 25).
Here, we report increased expression levels of PD-L1 on EBTr cells, EBL cells, and BAL-Mϕ after M. bovis infection as determined by flow cytometry (FCM) and by immunofluorescence (IF) staining of lung tissues. Additionally, we analyzed the differentially modulated subpopulations having intermediate and high PD-1 expression levels on both CD8+ and CD4+ T cells after M. bovis infection of cattle PBMCs, that suggesting T-cell exhaustion is linked to significant decreases in CD4+ PD-1INT and CD8+ PD-1INT subsets and significant increases in CD4+ PD-1HIGH and CD8+ PD-1HIGH subsets. Finally, we demonstrated the restoration of the proliferation of Mb1-infected PBMCs following a disruption of the PD-1/PD-L1 interaction using blocking anti-PD-1 antibodies. Our data will help in the development of future vaccine and therapeutic strategies to eliminate the PD-1-related immune exhaustion of T cells during M. bovis infection.
RESULTS
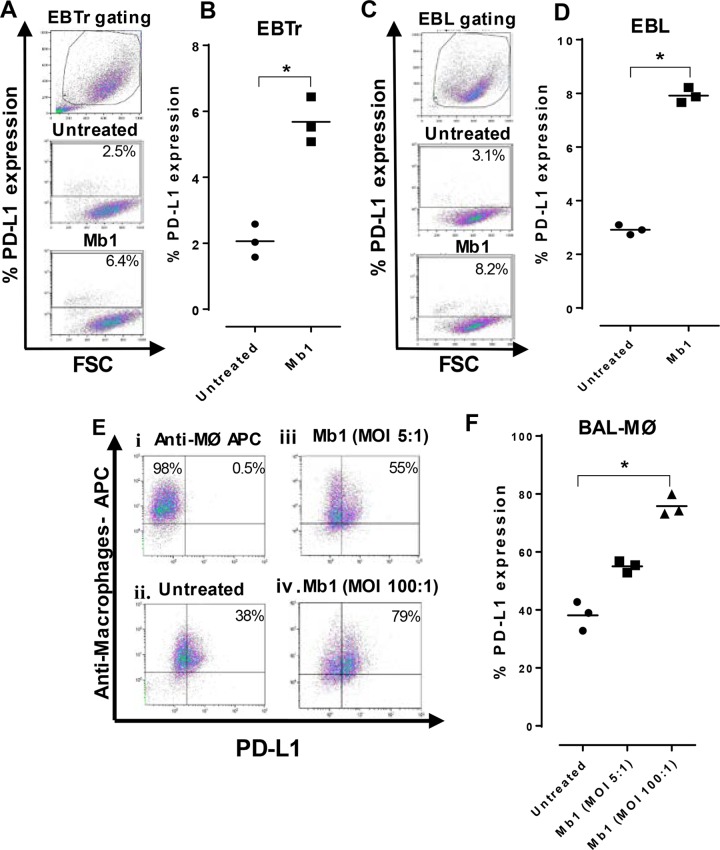
M. bovis infection augments PD-L1 expression on EBTr and EBL cell lines and BAL-Mϕ.
Our group previously described the invasion of EBTr and EBL cell lines by Mb1 (6) and found maximum invasion of both cell lines by Mb1 at 18 h of incubation. Therefore, we decided to test the expression of PD-L1 at maximum invasion 18 h after incubation with Mb1. Compared to the untreated controls, we found a significant increase (P < 0.05) in the expression level of PD-L1 in EBTr (Fig. 1A and B) and EBL (Fig. 1C and D) cell lines. The surface expression of PD-L1 was tested on BAL-Mϕ after 18 h of incubation under untreated conditions, and we found that 38% of BAL-Mϕ expressed PD-L1 (Fig. 1Eii). Furthermore, we observed that the cell surface expression of PD-L1 was increased to 55% (Fig. 1Eiii) and 79% (Fig. 1Eiv) in BAL-Mϕ infected at two different multiplicities of infection (MOIs) of 5:1 and 100:1, respectively. A significant increase (P < 0.05) in PD-L1 expression was observed at an MOI of 100:1 compared to untreated BAL-Mϕ (Fig. 1F).
FIG 1.
PD-L1 expression on EBTr and EBL cell lines and BAL-Mϕ is increased after M. bovis infection. PD-L1 expression on different cells was tested after 18 h of incubation with or without M. bovis infection at different MOIs. (A, C, and E) FCM gating on EBTr cells (A), EBL cells (C), and BAL-Mϕ (E) showing different conditions, including untreated and Mb1 treated cells. (B, D, and F) Graphs showing percentages of PD-L1 expression on EBTr cells (B), EBL cells (D), and BAL-Mϕ (F). There was a significant increase in PD-L1 expression after Mb1 infection at an MOI of 100:1. Three technical replicates were always performed under every condition in each experiment, and the graphs indicate the medians and ranges of representative data from one of three independent experiments. Panel F shows representative data for BAL-Mϕ collected from 3 cattle separately in three independent experiments. *, P < 0.05 for significant differences in PD-L1 expression on Mb1-infected cells versus untreated cells. APC, allophycocyanin.
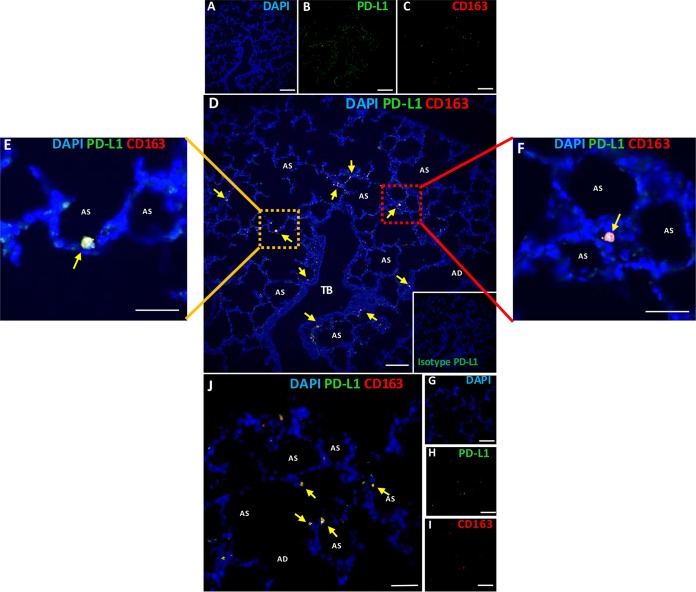
PD-L1 localizes to lung epithelial cells and alveolar macrophages in experimentally infected cattle.
In alignment with the in vitro studies, we proceeded to evaluate if PD-L1 was indeed expressed in pulmonary tissue following M. bovis infection. We localized PD-L1 in lung tissue obtained from cattle experimentally infected with M. bovis by IF analysis. Representative IF images from one control animal and one infected animal are shown in Fig. 2. We know from our previous experience with multiple animal trials that lung tissues become infected by M. bovis after experimental intranasal inoculation with a high challenge dose (7, 8, 32). Our group demonstrated that M. bovis inoculation not only makes cattle clinically sick but also generates specific lesions in lung tissue in response to experimental challenge (32). Hence, we performed double-IF staining of lung tissues from these experimentally challenged cattle to localize PD-L1 on the bovine lung epithelium as well as on alveolar Mϕ. We found that PD-L1 was localized in lung epithelial cells as well as on alveolar Mϕ (Fig. 2B). Furthermore, we identified alveolar Mϕ using antibody against the CD163 receptor (Fig. 2C) and colocalized IF staining in the lung tissue along with merged DAPI (4′,6-diamidino-2-phenylindole), PD-L1, and CD163 staining (Fig. 2D). A higher magnification of two areas of interest showed PD-L1+ CD163+ macrophages and some PD-L1+ lung epithelial cells (Fig. 2E and F). We also processed control lung tissue sections (not infected by Mb1) and stained them with PD-L1 and CD163 antibodies; single stainings with DAPI and PD-L1 and CD163 antibodies are shown in Fig. 2G to I, respectively. The representative merged image for the control section (Fig. 2J) shows basal expression and localization of PD-L1+ CD163+ macrophages and PD-L1+ lung epithelial cells.
FIG 2.
Localization of PD-L1 in bovine lung epithelial cells and alveolar macrophages of control and experimentally infected cattle. Lung tissues from one control and three infected cattle were processed for IF localization of PD-L1. (A to C) Multicolor IF staining of infected lung sections with DAPI for nuclei (blue), the immunoinhibitory ligand PD-L1 (green), and CD163 for alveolar macrophages (red). (D) Merge of panels A to C, with yellow arrows pointing at the colocalization of alveolar macrophage staining for CD163 and PD-L1. (E and F) Zoom images from panel D showing higher magnifications of alveolar macrophages. (G to J) IF staining of control uninfected lung sections for CD163 and PD-L1. Bars, 100 μm (A to D and G to J) and 10 μm (E and F). TB, terminal bronchiole; AD, air duct; AS, air sac.
M. bovis infection upregulates the expression of PD-1 on total PBMCs and CD4+ and CD8+ T cells.
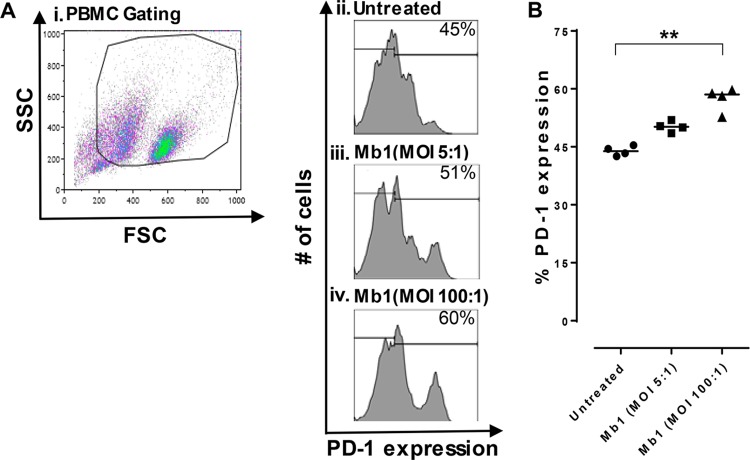
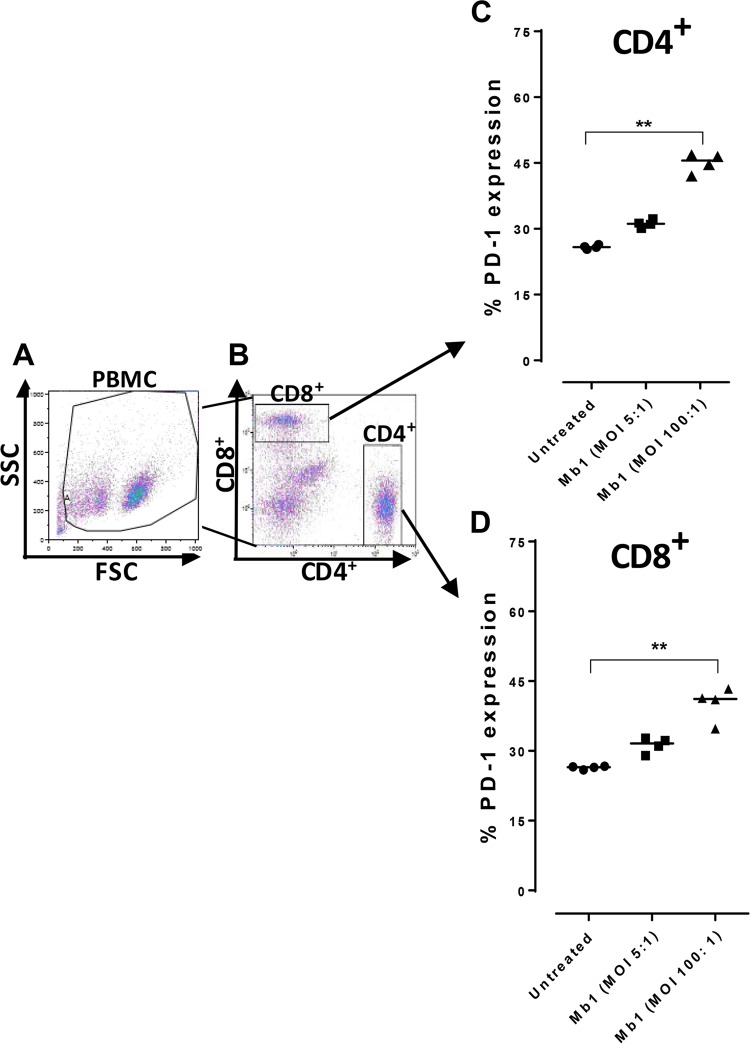
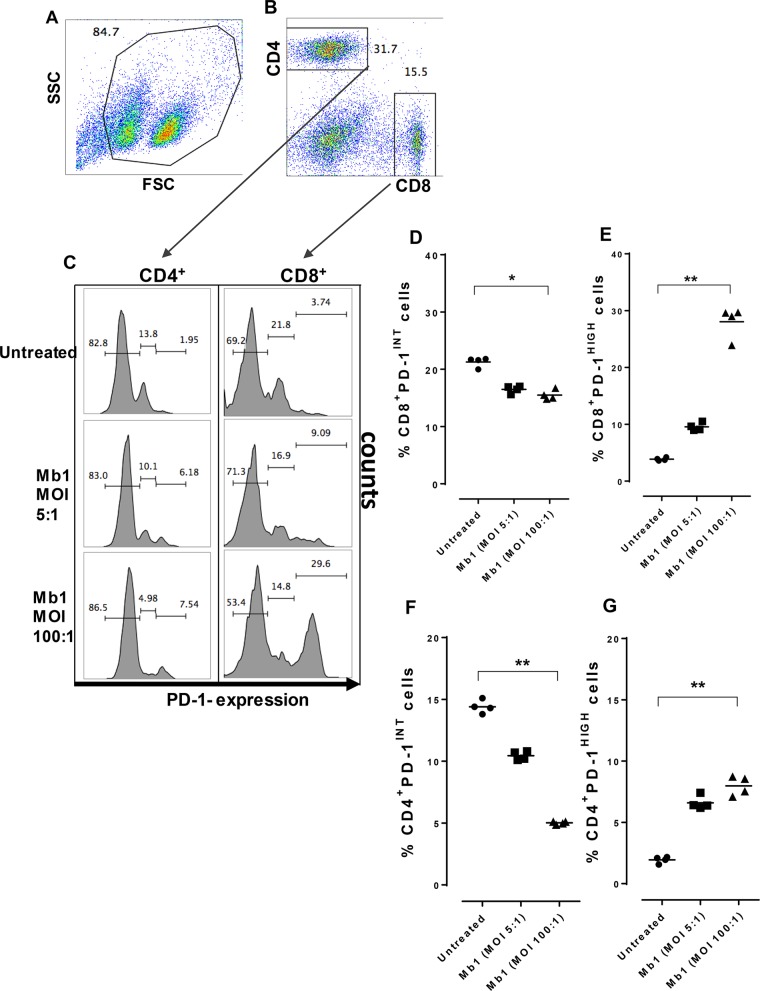
Our previous findings with cattle PBMCs showed that M. bovis has the capacity to efficiently infect bovine PBMC populations, leading to an inhibition of their proliferation (6, 28). A recent study showed increased PD-1 levels in cattle diagnosed with mycoplasmosis (24). It was therefore necessary to further explore infection of bovine total PBMC populations by Mb1 at different MOIs (5:1 and 100:1). We found that an MOI of 5:1 led to a 51% increase in PD-1 expression (Fig. 3Aiii), with a further increase to 60% (P < 0.01) (Fig. 3B) at an MOI of 100:1 (Fig. 3Aiv), whereas untreated PBMCs exhibited basal expression of PD-1 (Fig. 3Aii), at 45%. We further investigated T-cell exhaustion caused by the cell surface upregulation of PD-1 on CD4+ and CD8+ T-cell populations using FCM. PBMCs were infected in vitro with Mb1 for 18 h, and FCM was performed after gating for CD4+ and CD8+ T cells using specific antibodies (Fig. 4A and B). Increased expression levels of PD-1 were observed in both CD4+ and CD8+ T cells (Fig. 4C and D) (P < 0.01).
FIG 3.
PD-1 expression is increased on bovine PBMCs after M. bovis infection. PD-1 expression on PBMCs was tested after 18 h of incubation with or without M. bovis at different MOIs. (A) FCM gating on PBMCs showing different conditions, including untreated cells and Mb1-treated cells at different MOIs (5:1 and 100:1). SSC, side scatter; FSC, forward scatter. (B) Percent PD-L1 expression on PBMCs showing significant increases in PD-1 expression after Mb1 infection at an MOI of 100:1. Three technical replicates were always performed under every condition in each experiment, and the graphs indicate the medians and ranges of representative data from one of three independent experiments (four animals in each experiment). **, P < 0.01, representing significant differences in PD-1 expression on Mb1-infected PBMCs versus untreated cells.
FIG 4.
M. bovis infection of CD4+ and CD8+ bovine T cells increases expression of the immunoinhibitory receptor PD-1. (A and B) PBMCs were gated and sorted to study CD4+ and CD8+ T cells for PD-1 expression after 18 h of incubation with or without M. bovis at different MOIs. (C) Results showing percentages of PD-1 expression on CD4+ T cells, with significant increases after Mb1 infection at an MOI of 100:1. (D) Percentages of PD-1 expression on CD8+ T cells also show a significant increase after Mb1 infection at an MOI of 100:1. Three technical replicates were always performed under every condition in each experiment, and the graphs indicate the medians and ranges of representative data from one of three independent experiments (four animals in each experiment). **, P < 0.01, representing significant differences in PD-1 expression on Mb1-infected cells versus untreated cells.
CD4+ PD-1INT and CD8+ PD-1INT subsets serve as progenitors giving rise to CD4+ PD-1HIGH and CD8+ PD-1HIGH subsets.
We sought to determine the origins of the high-PD-1+-expressing CD4+ and CD8+ T cells during the course of an established chronic infection such as mycoplasmosis in cattle. We therefore investigated the level of PD-1 expression on both T cell populations before and after Mb1 infection at different MOIs (5:1 and 100:1) (Fig. 5A and B) and found distinct subsets of cells expressing intermediate and high levels of PD-1+ expression, as described previously by others (17) (Fig. 5C). Upon further analysis, we observed gradual decreases in the cell populations of CD4+ PD-1INT and CD8+ PD-1INT subsets (Fig. 5D and F) and their function as progenitor pools rather giving rise to CD4+ PD-1HIGH and CD8+ PD-1HIGH subsets (Fig. 5E and G). Significant differences in this transition were observed at an MOI of 100:1; however, a similar trend was observed at an MOI of 5:1.
FIG 5.
PD-1INT T-cell subsets function as progenitor pools for PD-1HIGH T-cell subsets. The pools of exhausted T cells were analyzed to identify pre- and postinfection changes in CD4+ PD-1INT and CD8+ PD-1INT subsets and also CD4+ PD-1HIGH and CD8+ PD-1HIGH subsets. (A and B) PBMCs were gated and sorted to study CD4+ and CD8+ T cells for PD-1 expression after 18 h of incubation with or without M. bovis at different MOIs. (C) Results showing percentages of PD-1 expression on CD4+ T cells and CD8+ T cells in the control and after Mb1 infection at MOIs of 5:1 and 100:1 in distinct intermediate and high subsets based on PD-1 expression. (D) Significant decrease in the percentages of CD8+ PD-1INT cells after Mb1 infection (MOI of 100:1) (**, P < 0.05). (E) Significant increase in the percentages of CD8+ PD-1HIGH cells after Mb1 infection (MOI of 100:1) (**, P < 0.01). (F) Significant decrease in the percentages of CD4+ PD-1INT cells after Mb1 infection (MOI of 100:1) (**, P < 0.01). (G) Significant increase in the percentages of CD4+ PD-1HIGH cells after Mb1 infection (MOI of 100:1) (**, P < 0.01). Three technical replicates were always performed under every condition in each experiment, and the graphs indicate the medians and ranges of representative data from one of three independent experiments (four animals in each experiment).
M. bovis infection induces temporal inhibition of bovine PBMC proliferation.
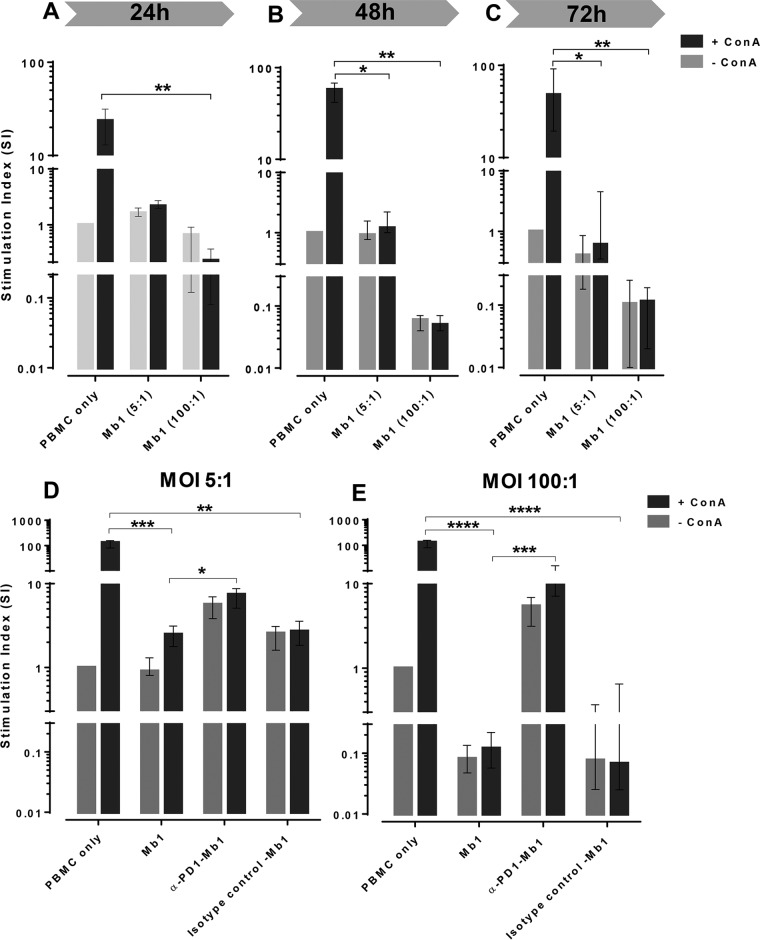
In order to investigate the inhibitory effects of Mb1 on the expansion capacity of PBMCs, we performed proliferation assays as described previously (6). There was significant inhibition (P < 0.01) when the stimulation indexes (SIs) of ConA-stimulated cells treated or untreated with Mb1 were compared, which started as early as 24 h postinfection (Fig. 6A). Inhibition of PBMC proliferation (P < 0.05) was observed at an MOI of 5:1 starting at 48 h (Fig. 6B) and also at 72 h (Fig. 6C). The inhibition caused by Mb1 at an MOI of 100:1 persisted significantly at 48 h and 72 h (Fig. 6B and C), indicating a mechanism of inhibition mediated by constant inhibitory signaling to PBMCs, leading to their nonproliferative exhaustion.
FIG 6.
Temporal inhibition of PBMC proliferation after M. bovis infection and its reactivation after PD-1 blockade. Bovine PBMCs were incubated with Mb1, and incubations were performed for 24, 48, and 72 h to evaluate the temporal inhibition of proliferation under ConA-stimulated conditions. (A to C) Significant proliferation inhibition after incubation for 24 h for ConA-stimulated PBMCs without versus those with Mb1 infection (100:1) (A) and significant proliferation inhibition after 48 h of incubation for ConA-stimulated PBMCs without versus those with Mb1 infection (MOIs of both 5:1 and 100:1) (B and C). (D) Blockade of PD-1 by anti-PD-1 antibody over the course of infection by Mb1 leads to significant restoration of proliferation at an MOI of 5:1. (E) Similar restoration of PBMC proliferation at an MOI of 100:1. No effects were seen when cells were incubated with an isotype control. Shown are representative data for PBMCs collected from 4 animals in three independent experiments. Every individual animal had each treatments done in triplicates, and the bar graphs show representative data as medians and ranges for four animals. Significant results are shown (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 [for non-ConA versus ConA treatments]).
M. bovis-induced inhibition of PBMC proliferation is ameliorated after blocking PD-1.
To determine whether the inhibition of PBMC proliferation, as seen in previous studies (6, 8, 28), is dependent on the PD-1 immunoinhibitory pathway, we incubated PBMCs with unconjugated anti-PD-1 antibody to block the PD-1 receptors. The treatments included incubation with Mb1 and an anti-PD-1 antibody or an isotype control from the start of the assays to block PD-1 during infection until the final harvest of the cells. The experiment was performed by using Mb1 at two different MOIs, 5:1 (Fig. 6D) and 100:1 (Fig. 6E). We observed the classical inhibition of the proliferation of ConA-stimulated PBMCs with Mb1, which was significantly different (P < 0.001) from that under untreated (PBMCs only) ConA-stimulated conditions, whereas blockade with an anti-PD-1 antibody led to a limited yet significant (P < 0.05) restoration of the proliferation of PBMCs infected with Mb1 at an MOI of 5:1. At the same time, infected PBMCs incubated with an isotype control antibody showed significantly inhibited proliferation (P < 0.01) (Fig. 6D). A similar experiment was performed by using a higher MOI of 100:1. Compared to untreated (PBMCs only) ConA-stimulated conditions, we observed a more significant inhibition of the proliferation of ConA-stimulated PBMCs after incubation with Mb1 (P < 0.0001) (Fig. 6E). In addition, a significant restoration of the proliferative properties of PBMCs was observed after blockade with an anti-PD-1 antibody (P < 0.001). Moreover, restoration of proliferation was not seen after using a specific isotype control antibody (Fig. 6E).
DISCUSSION
The control of the PD-1/PD-L1 immune checkpoint is an important mechanism that has been shown to remarkably modulate the adaptive immune response. In this study, we have dissected how M. bovis regulates and exploits the PD-1/PD-L1 immune checkpoint on multiple cell types to escape cell-mediated immunity. We further demonstrate that the induction of this axis impairs the proliferative capacity of T cells. Finally, we demonstrate the functional role of PD-L1-blocking antibody in disrupting the PD-1/PD-L1 axis, thereby reversing the inhibitory effect on the T-cell-proliferative capacity induced by M. bovis infection.
Over recent years, M. bovis has had a significant impact on the cattle industry due to its chronic morbidity and significant mortality. This wall-less bacterial pathogen has been known to infect both cattle and bison, causing bronchopneumonia, arthritis, otitis, mastitis, reproductive disorders, meningitis, and conjunctivitis (33). In contrast to its increased prevalence and distribution, mycoplasmal infections are accompanied by a limited understanding of the mechanisms of disease resistance (34). Additionally, several strains that have been isolated from multiple outbreaks necessitate further inspection to understand the fundamental molecular basis of this disease (35).
Effective vaccination strategies can be derived from dissecting the mechanistic aspects involved in M. bovis pathogenesis. An inability to recruit immune cells, impaired clonal expansion, a loss of effector functional capacity, and the exhaustion phenotype all appear to limit protection conferred by cell-mediated immunity, leading to persistent infections culminating in chronicity and perhaps an increased incidence of tumor/nodule development (36). Inhibitory receptors on T cells belonging to the CD28 family have been associated with impaired cell-mediated immunity. Changes in the expression level of PD-1, a key receptor of the CD28 family, play an important role in modulating the immune responses to intracellular infections, with different types of viruses exploiting this pathway in order to escape immune surveillance (37).
There have been several observations that indicate impaired lymphocyte proliferation in M. bovis infections, with multiple speculations. Prior to the discovery of VSPs, a family of surface proteins of M. bovis, it has been shown that some degree of lymphocyte proliferation is inhibited in response to lectin mitogens with M. bovis infection (38). Previous results from our laboratory, studying the effect of Mb1 on lymphocyte proliferation (6), were consistent with data from a study by Thomas et al. (39), which showed that M. bovis-infected cultures suppress phytohemagglutinin (a nonspecific mitogen)-stimulated lymphocyte responses in a dose- and time-dependent manner. This observed suppression was not due to differences in cell viability (28, 29). Previous in vitro studies by Naot et al. (40) similarly investigated lymphocyte responses to phytohemagglutinin, demonstrating a decreased proliferative capacity of lymphocytes in cultures inoculated with various Mycoplasma strains and species.
In line with the above-mentioned observations, we set out to investigate the source of PD-L1, which may have implications in suppressing cell-mediated immunity. As it is well known that M. bovis infection infects the lungs prior to systemic dissemination, we wanted to assess its impact on cells of the pulmonary microenvironment. To this end, our first set of in vitro studies exhibited promising results for PD-L1 induction in EBTr and EBL cell lines (Fig. 1B and D). We further investigated the induction of the PD-1 ligand on ex vivo cattle-derived pulmonary macrophages. The Mb1 dose-dependent increase in PD-L1 expression that was observed in these macrophages (Fig. 1F) further incited us to affirm these findings in an actual disease setting. Accordingly, we confirmed these observations via conducting immunofluorescence analysis of necropsied pulmonary samples obtained from infected cattle (Fig. 2). In alignment with our postulate, the in vivo data endorse our in vitro and ex vivo model where M. bovis induced PD-L1 in key cells in bovine lungs. It was shown previously that M. bovis is known to infect hosts both intracellularly and extracellularly (41). Bovine lung macrophages are vital for capturing invading M. bovis in lung tissues, and we have shown that M. bovis is capable of not only infecting these macrophages but also surviving in them (6). Our results demonstrated that the expression level of PD-L1 was increased in Mb1-infected EBTr and EBL cell lines and in BAL-Mϕ at an MOI of 100:1 compared to untreated controls. The cells that have increased expression levels of PD-L1 are also the same cells that are the target for infection by Mb1. This novel immune evasion mechanism may well prove to be the fundamental cause of the prolonged pathogenicity of M. bovis. Moreover, the different effects of Mb1 at MOIs of 50:1 and 100:1 suggest a dose-dependent induction of PD-1 that may be a factor contributing to variable disease outcomes. In addition, the different virulent strains of M. bovis may account for the differential induction of PD-1 and its ligands, which may influence the disease outcome.
Although our experimental observations clearly delineated the induction of PD-L1 in multiple cell types in pulmonary tissue following exposure to M. bovis (Mb1), we still were unable to ascertain its immunomodulatory role. In order to exert its effects, the ligand needs to engage its cognate receptor PD-1 on target cells. Multiple reports of impaired cell-mediated responses in M. bovis infections prompted us to analyze its impact on the lymphocytic population. It is well documented that PD-1 is induced following T-cell receptor signaling, and perhaps, its cell surface expression on activated T cells is necessary to prevent runaway immune responses (42). The engagement of PD-1 by its ligand (PD-L1 or -L2) inhibits proliferation and induces apoptosis in T cells (43). Under regular conditions, it is considered a safeguard against exaggerated immune responses.
Conversely, we hypothesized that the PD-1/PD-L1 pathway may be a crucial mechanism for immune escape if M. bovis infection induces PD-1 on effector T cells. Consistent with our hypothesis, we observed an increase in the cell surface expression of PD-1 on stimulated PBMCs that were exposed to Mb1 compared to the control population (Fig. 3). We further investigated whether this observed induction impacted helper T cells or cytotoxic T cells. Flow cytometric analysis yielded interesting results, where both CD4+ and CD8+ T cells were identified as target populations that had increased numbers of PD-1-positive cells (Fig. 4).
Chronic infection with M. bovis indicates the long-term exhaustion of immune cells, particularly CD4+ and CD8+ T cells. This phenomenon has additionally been reported for other chronic infections such as LCMV infections (18, 19) and chronic stages of bovine Fasciola hepatica infections (44). PD-1 expression levels in CD4+ and CD8+ T-cell subsets may have a significant role in progression to persistent chronic infections. Others have demonstrated that T cells having intermediate levels of PD-1 expression (CD4+ PD-1INT and CD8+ PD-1INT subsets) are progenitors of the pool giving rise to the terminally differentiated subsets having high levels of PD-1 expression (CD4+ PD-1HIGH and CD8+ PD-1HIGH subsets) (17). Interestingly, our findings show for the first time that M. bovis infection leads to a decline in CD4+ PD-1INT and CD8+ PD-1INT subsets with a concomitant increase in both the CD4+ PD-1HIGH and CD8+ PD-1HIGH subsets. Diminished mitochondrial and glycolytic metabolisms in CD4+ PD-1HIGH and CD8+ PD-1HIGH subsets are the key factors for diminished effector functions needed by the cells to perform optimally and counteract infection (18). A closer look at the data suggests a substantial increase in the CD8+ PD-1HIGH population compared to the CD4+ PD-1HIGH population, indicting that the effector cytotoxic T-cell response may be more significantly impaired in a high-infectivity model (Fig. 5). Infections by Mannheimia haemolytica, Histophilus somni, and Pasteurella multocida have commonly been reported with M. bovis infections (45, 46). The observation of the nonspecific expression of PD-1 on T cells that may disrupt their functionality may be one of the factors sustaining the coinfections observed in these cases. The pathogenic impact of M. bovis infection is also suggestive of impaired memory T-cell responses, which necessitates further investigation.
The induction of the PD-1/PD-L1 axis has multiple immunosuppressive effects; however, the inhibition of T-cell proliferation is one of the hallmarks of this pathway. Hence, to confirm the functional outcome, we assessed the proliferative capacity of T cells under the influence of Mb1. As expected, the proliferation response of PBMCs was inhibited significantly in a dose-dependent manner, which correlated well with the induction of CD4+ PD-1HIGH and CD8+ PD-1HIGH subsets as described above. This loss of proliferative capacity may be due to other suppressive pathways. To affirm the hypothesis that the inhibition of PBMC proliferation and exhaustion of T cells by Mb1 infection are associated with the observed upregulation of the immunoinhibitory receptor PD-1 and its ligand, we blocked the PD-1/PD ligand interaction and examined the functional restoration of antigen-specific PBMC proliferation in vitro, which was clearly demonstrated at an MOI of 100:1. Although the control sample with anti-PD-1 antibody-treated cells exhibited a mild, nonsignificant population expansion, we speculate that it could be due to the unopposed proliferative signaling in these cells and linked to autoimmune responses (47). Although not assessed directly, decreased T-helper-cell expansion could negatively influence the cytotoxic T-cell responses. In addition, interference in the helper population could impact B-cell responses, which may be of interest for future studies.
In light of these findings, we propose that M. bovis utilizes this pathway as a fundamental mechanism for immune evasion by targeting immune effectors. However, it is a valid assumption that other inhibitory pathways, such as the cytotoxic-T-lymphocyte-associated antigen 4 (CTLA-4), indoleamine 2,3-dioxygenase (IDO), Cbl-b, transforming growth factor β (TGF-β), and interleukin-10 (IL-10) pathways, might contribute to the development of an immune inhibition status. Interestingly, our finding that the antibody-mediated blocking of PD-1 showed a reversal of the proliferative capacity of PBMCs clearly demonstrates the irrefutable role of PD-1/PD-L1 in this process. Although previous studies by Kinter et al. (48) demonstrate the role of common gamma chain cytokines in the induction of PD-1, the present study was not designed to explore the role of cytokine responses that may be involved in the induction of PD-1. Our results, however, indicated that the PD-1/PD-L1 axis is induced in multiple different cell types following M. bovis infection, suggesting a rather direct role of the pathogen in orchestrating the PD-1/PD-L1 axis. The molecular pathways that are involved in PD-1/PD-L1 axis regulation by M. bovis are of significant interest for future studies, and the underlying molecular signaling pathways induced by M. bovis infection may well prove to be targets for interventional therapy. It has been suggested that reinvigorating the cells with intermediate levels of PD-1 expression (CD4+ PD-1INT and CD8+ PD-1INT subsets) could be the key to effective T-cell functions. Early disease detection followed by immune intervention may serve to reverse or limit the disease process and is an interesting area that will be the focus of future therapeutic trials. In addition, antibody-mediated therapy for the PD-1/PD-L1 checkpoint as an adjunct to regular vaccines may provide a rapid solution for vaccination and have a positive impact on the current disease burden, especially when such strategies have proven to be beneficial in other infection models (18, 19, 27). These results imply that Mb1 orchestrates the PD-1 pathway to depress the proliferative capacity of immune cells, thereby eluding immune detection and resulting in a delayed cell-mediated immune response, which possibly renders the host incapable of mounting an effective immune response.
MATERIALS AND METHODS
Bacterial stock and media.
M. bovis strain Mb1, used in all experiments, was isolated from the synovial fluid of a calf showing signs of arthritis (49). Cultures of Mb1 were grown in modified Hayflick's broth at 37°C with 5% CO2. Bacterial cells were collected at the exponential phase of growth and were centrifuged at 5,500 × g for 15 min, followed by washing with minimum essential medium (MEM; Invitrogen, Burlington, ON, Canada). Mb1 bacteria were stocked at −70°C in MEM (supplemented with 30% glycerol) at a concentration of 108 CFU/ml.
Cell lines and media.
EBTr and EBL cells were obtained from the ATCC (NBL-4; ATCC CCL-44) and the DSMZ (ACC 192), respectively. Eagle's minimum essential medium (EMEM) (Sigma Life Science) supplemented with 10% fetal bovine serum (FBS), 100 U/ml of penicillin, and 100 μg/ml streptomycin was used for EBTr cultures, whereas RPMI 1640 supplemented with 15% FBS, 100 U/ml of penicillin, and 100 μg/ml streptomycin was used for cultures of the EBL cell line. Both cell lines were cultured as described previously (6). We evaluated cell viability using a trypan blue exclusion method as described previously (28).
PBMC purification and culture.
Bovine PBMCs were isolated from blood collected in EDTA-coated Vacutainer tubes from cattle with no history of M. bovis or other bacterial infections at the VIDO-InterVac research farm. PBMCs were isolated as described previously (6). Briefly, cells were separated by centrifugation (2,500 × g for 20 min), and the buffy coat fraction containing PBMCs was purified by using Ficoll gradients (GE Healthcare, Mississauga, ON, Canada). Culturing was done by using EMEM supplemented with 10% FBS, 0.5 ml of 2-mercaptoethanol (50 mM), and 5 ml each of nonessential amino acids (NEAA), sodium pyruvate (100 mM), and 1 M HEPES solution but without the addition of antibiotics. PBMCs were washed three times with phosphate-buffered saline (PBS), followed by centrifugation (800 × g for 5 min). Cell viability was determined by using the trypan blue exclusion method as described above for EBTr and EBL cell lines. Moreover, all PBMC samples were plated and incubated at 37°C for at least 5 days on Hayflick's agar (HFA) and blood agar for the detection of any M. bovis infection or contamination. The cell samples were used only if they were M. bovis free as indicated by negative cultures.
PBMC proliferation assay.
The PBMC proliferation assay was performed as described previously (6, 28). Briefly, PBMCs from individual animals were plated separately at a cell density of 3.5 × 105 PBMCs/well in 96-well U-bottom plates (Thermo Scientific, Denmark) in triplicates following counting on a Coulter counter, and subsequent aliquots were also collected to determine the viability of PBMCs by a trypan blue exclusion assay. PBMCs were infected with Mb1 at MOIs of 5:1 and 100:1 (Mb1 bacteria/PBMCs). We used ConA (1 μg/ml) as a positive control following 72 h of incubation (37°C with 5% CO2). In addition, proliferation kinetics were conducted in triplicates at 24, 48, and 72 h. An additional incubation for 18 h was carried out under all conditions after the addition of [3H]thymidine (0.4 μCi/well), PBMCs were harvested (Filtermate harvester; Packard), and a scintillation counter (Top Count NXT; Packard) was used to quantify incorporated [3H]thymidine. Proliferation results were measured as counts per minute, shown as SIs, using the following formula: counts per minute of treated PBMCs/counts per minute of control or unstimulated PBMCs.
BAL-Mϕ purification and culture.
Lung lavage fluids were obtained from a certified local abattoir slaughtering inspected healthy cattle, and BAL-Mϕ were purified as described previously (6). Briefly, 4 liters of Hanks' buffered saline solution with chloramphenicol (HBSS-CMF) containing an antibiotic-antimycotic cocktail, 10,000 U/ml of penicillin, 10 mg/ml of streptomycin, 25 μg/ml of amphotericin B (Sigma, USA), and 100 μg/ml of gentamicin (Gibco, USA) was used for lung lavage. The lavage fluid was filtered by using a 20-μm-pore fabric filter, centrifuged for 10 min (400 × g at 4°C), and washed twice by using cold sterile PBS (with antibiotic-antimycotic solution). Dulbecco's modified Eagle medium (DMEM) supplemented with 2% FBS, 14 mM HEPES, 0.1 mM nonessential amino acids, 5 mg/ml glucose, 4 mM l-glutamine, 100 IU/ml sodium penicillin G (Gibco, USA), and an antibiotic-antimycotic solution was used when needed. The cells were incubated for 3 h at 37°C with 5% CO2 to adhere macrophages to the plates, the supernatant was then gently removed, and new prewarmed culture medium (without antibiotics) was added. We tested the purity of purified BAL-Mϕ by FCM using a FACSCalibur instrument after staining with the macrophage-specific monoclonal antibody (Serotec) (see Table S1 in the supplemental material) and found the purity of BAL-Mϕ to be >98% (Fig. 1Ei). We plated purified samples of BAL-Mϕ on HFA and blood agar to exclude contaminated samples during collection and isolation. Samples were always used from animals that were M. bovis free as indicated by negative cultures.
PD-L1 expression on EBTr cells, EBL cells, and BAL-Mϕ.
PD-L1 expression after exposure to M. bovis was determined on different cells (EBTr cells, EBL cells, and BAL-Mϕ). Flat-bottom 6-well plates (Costar; Corning Inc., NY) were used, and 1 × 106 cells were infected individually with Mb1 at two different MOIs (5:1 and 100:1) for BAL-Mϕ and at an MOI of 100:1 for EBTr and EBL cells. Uninfected control and Mb1-infected cells were incubated for 18 h at 37°C with 5% CO2. Cells were then harvested by using trypsin as previously described (6) and washed twice with PBS. Cells were incubated on ice with anti-PD-L1 antibody (Santa Cruz Biotechnology Inc.) or an isotype control at a 1:50 dilution for 30 min and later washed three times by using 200 μl PBS–0.03% sodium azide (EMD Chemicals, USA). Fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit isotype-specific secondary antibody was added (1 μg/ml), and the mixture was incubated for 30 min on ice in the dark. The cells were washed three times with PBS–0.03% sodium azide, followed by a fixation step using 2% formaldehyde (Sigma-Aldrich). A FACSCalibur instrument (Becton Dickinson, Franklin Lakes, NJ) was used for sample acquisition, and 20,000 events were acquired with Cell Quest software (version 3.3). Later, data were analyzed by using Kaluza software (v. 1.3).
PD-1 expression on total PBMCs, CD4+ T cells, and CD8+ T cells.
PD-1 expression was detected by using the FITC-conjugated anti-PD-1 antibody (Biorybt Ltd., UK). Briefly, purified PBMCs (5 × 105 cells/well) were incubated for 18 h at 37°C with 5% CO2 with or without Mb1 at two different MOIs (5:1 and 100:1). Cells were then centrifuged, suspended in cold PBS for labeling with anti-PD-1 antibody and isotype control FITC-conjugated rabbit IgG antibody (Santa Cruz Inc., USA) at a 1:50 dilution, incubated for 30 min on ice in the dark followed by two washes with PBS–0.03% sodium azide, and analyzed by flow cytometry. For further evaluation of PD-1 expression on subpopulations of PBMCs, we performed an experiment similar to the one described above and double stained PBMCs with anti-CD8+ (1:100 dilution) and anti-CD4+ (1:200 dilution) antibodies (VMRD/Kingfisher, USA), followed by their corresponding secondary fluorescently labeled antibodies at a 1:100 dilution (see Table S1 in the supplemental material). Flow cytometry was performed, 20,000 events were acquired, and data were analyzed by using Kaluza and FlowJo software.
Effect on PBMC proliferation after blocking PD-1.
The assay to block PD-1 expression on PBMCs was carried out by using an unconjugated anti-PD-1 antibody (OriGene Technologies Inc., USA). The assay was carried out as mentioned above for the PBMC proliferation assay, with a few modifications. Briefly, PBMCs were plated (3.5 × 105 PBMCs/well) in triplicates and either infected by Mb1 at an MOI of 5:1 or 100:1 or stimulated with ConA (1 μg/ml) as a positive control, followed by 72 h of incubation (37°C with 5% CO2). PBMCs without any stimulation by ConA and without any M. bovis infection were used as negative controls. At the time of infection with Mb1, cells in paired wells were treated with unconjugated anti-PD-1 antibody (20 μg/ml) or an isotype control antibody during incubation. Cells were then incubated for 18 h after the addition of [3H]thymidine (0.4 μCi/well), and PBMCs were harvested. Proliferation results are shown as SIs.
Immunofluorescence detection of PD-L1 in lung tissue samples from experimentally infected cattle.
Over the years, our research group has developed a highly efficient and reliable M. bovis challenge model in cattle for studying in vivo immune responses and testing different experimental vaccines (32). We tested lung tissue samples obtained from one naive or three M. bovis-challenged animals, and the tissues were formalin fixed, embedded in paraffin wax, and then cut into 5-μm-thick sections. The dried sections were deparaffinized in xylene, and antigen retrieval was performed in citrate buffer (0.37 g/liter citric acid and 2.4 g/liter trisodium citrate dihydrate) in a water bath at 95°C for 10 min. The slides were washed with PBS for 5 min and incubated for 1 h with a multispecies 10% serum blocking solution, and all subsequent incubations were done in the dark. Primary anti-PD-L1 antibody (see Table S1 in the supplemental material) was used at a 1:200 dilution, and the sections were incubated for 1 h at room temperature. An isotype control was used in parallel on similar tissue sections under similar conditions, followed by three washes in cold PBS for 5 min each. A secondary antibody linked with FITC was added at a 1:400 dilution for 1 h at room temperature. The sections were washed in cold PBS for 5 min each, counterstaining was done with anti-CD163-phycoerythrin (PE) antibody (Table S1) at a 1:25 dilution, and the sections were stained for 1 h at room temperature. The sections were washed again in cold PBS for 5 min each and mounted with medium containing DAPI for staining nuclei. The images were obtained by using an Olympus microscope and analyzed by using ImageJ software (version 1.50i).
Statistics.
A nonparametric Friedman test with Dunn's multiple-comparison test was used to analyze differences between median values for each group. Differences were considered significant at a P value of ≤0.05. Data were analyzed by using GraphPad Prism 7.00 for Windows (GraphPad Software, La Jolla, CA, USA).
Supplementary Material
ACKNOWLEDGMENTS
This work was made possible by financial support from the Alberta Livestock and Meat Agency Ltd. and the Saskatchewan Agriculture Development Fund.
We also thank Don Wilson and the VIDO Animal Care Unit for their invaluable help in obtaining blood and lung lavage samples.
Footnotes
This article is published with permission of the Director of VIDO-InterVac as journal series number 803.
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00921-17.
REFERENCES
- 1.OIE. 2016. OIE-listed diseases, infections and infestations in force in 2016. OIE, Paris, France: http://www.oie.int/en/animal-health-in-the-world/oie-listed-diseases-2016/ Accessed 29 October 2016. [Google Scholar]
- 2.Nicholas RA. 2011. Bovine mycoplasmosis: silent and deadly. Vet Rec 168:459–462. doi: 10.1136/vr.d2468. [DOI] [PubMed] [Google Scholar]
- 3.Caswell JL, Bateman KG, Cai HY, Castillo-Alcala F. 2010. Mycoplasma bovis in respiratory disease of feedlot cattle. Vet Clin North Am Food Anim Pract 26:365–379. doi: 10.1016/j.cvfa.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Nussbaum S, Lysnyansky I, Sachse K, Levisohn S, Yogev D. 2002. Extended repertoire of genes encoding variable surface lipoproteins in Mycoplasma bovis strains. Infect Immun 70:2220–2225. doi: 10.1128/IAI.70.4.2220-2225.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lerner U, Amram E, Ayling RD, Mikula I, Gerchman I, Harrus S, Teff D, Yogev D, Lysnyansky I. 2014. Acquired resistance to the 16-membered macrolides tylosin and tilmicosin by Mycoplasma bovis. Vet Microbiol 168:365–371. doi: 10.1016/j.vetmic.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 6.Suleman M, Prysliak T, Clarke K, Burrage P, Windeyer C, Perez-Casal J. 2016. Mycoplasma bovis isolates recovered from cattle and bison (Bison bison) show differential in vitro effects on PBMC proliferation, alveolar macrophage apoptosis and invasion of epithelial and immune cells. Vet Microbiol 186:28–36. doi: 10.1016/j.vetmic.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Prysliak T, van der Merwe J, Perez-Casal J. 2013. Vaccination with recombinant Mycoplasma bovis GAPDH results in a strong humoral immune response but does not protect feedlot cattle from an experimental challenge with M. bovis. Microb Pathog 55:1–8. doi: 10.1016/j.micpath.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Mulongo M, Prysliak T, Perez-Casal J. 2013. Vaccination of feedlot cattle with extracts and membrane fractions from two Mycoplasma bovis isolates results in strong humoral immune responses but does not protect against an experimental challenge. Vaccine 31:1406–1412. doi: 10.1016/j.vaccine.2012.12.055. [DOI] [PubMed] [Google Scholar]
- 9.Devi VR, Poumarat F, Le Grand D, Rosengarten R, Hermeyer K, Hewicker-Trautwein M. 2014. Histopathological findings, phenotyping of inflammatory cells, and expression of markers of nitritative injury in joint tissue samples from calves after vaccination and intraarticular challenge with Mycoplasma bovis strain 1067. Acta Vet Scand 56:45. doi: 10.1186/s13028-014-0045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mogensen TH. 2009. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev 22:240–273. doi: 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Avondt K, van Sorge NM, Meyaard L. 2015. Bacterial immune evasion through manipulation of host inhibitory immune signaling. PLoS Pathog 11:e1004644. doi: 10.1371/journal.ppat.1004644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. 2008. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. 2007. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol 8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 14.Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. 17 October 2016. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol doi: 10.1038/nri.2016.107. [DOI] [PubMed] [Google Scholar]
- 15.Okazaki T, Chikuma S, Iwai Y, Fagarasan S, Honjo T. 2013. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol 14:1212–1218. doi: 10.1038/ni.2762. [DOI] [PubMed] [Google Scholar]
- 16.Konnai S, Murata S, Ohashi K. 8 October 2016. Immune exhaustion during chronic infections in cattle. J Vet Med Sci doi: 10.1292/jvms.16-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paley MA, Kroy DC, Odorizzi PM, Johnnidis JB, Dolfi DV, Barnett BE, Bikoff EK, Robertson EJ, Lauer GM, Reiner SL, Wherry EJ. 2012. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science 338:1220–1225. doi: 10.1126/science.1229620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bengsch B, Johnson AL, Kurachi M, Odorizzi PM, Pauken KE, Attanasio J, Stelekati E, McLane LM, Paley MA, Delgoffe GM, Wherry EJ. 2016. Bioenergetic insufficiencies due to metabolic alterations regulated by the inhibitory receptor PD-1 are an early driver of CD8(+) T cell exhaustion. Immunity 45:358–373. doi: 10.1016/j.immuni.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blackburn SD, Shin H, Freeman GJ, Wherry EJ. 2008. Selective expansion of a subset of exhausted CD8 T cells by alphaPD-L1 blockade. Proc Natl Acad Sci U S A 105:15016–15021. doi: 10.1073/pnas.0801497105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Staples KJ, Nicholas B, McKendry RT, Spalluto CM, Wallington JC, Bragg CW, Robinson EC, Martin K, Djukanovic R, Wilkinson TM. 2015. Viral infection of human lung macrophages increases PDL1 expression via IFNbeta. PLoS One 10:e0121527. doi: 10.1371/journal.pone.0121527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hassan SS, Akram M, King EC, Dockrell HM, Cliff JM. 2015. PD-1, PD-L1 and PD-L2 gene expression on T-cells and natural killer cells declines in conjunction with a reduction in PD-1 protein during the intensive phase of tuberculosis treatment. PLoS One 10:e0137646. doi: 10.1371/journal.pone.0137646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh A, Dey AB, Mohan A, Mitra DK. 2014. Programmed death-1 receptor suppresses gamma-IFN producing NKT cells in human tuberculosis. Tuberculosis (Edinb) 94:197–206. doi: 10.1016/j.tube.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Ikebuchi R, Konnai S, Shirai T, Sunden Y, Murata S, Onuma M, Ohashi K. 2011. Increase of cells expressing PD-L1 in bovine leukemia virus infection and enhancement of anti-viral immune responses in vitro via PD-L1 blockade. Vet Res 42:103. doi: 10.1186/1297-9716-42-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goto S, Konnai S, Okagawa T, Nishimori A, Maekawa N, Gondaira S, Higuchi H, Koiwa M, Tajima M, Kohara J, Ogasawara S, Kato Y, Suzuki Y, Murata S, Ohashi K. 2017. Increase of cells expressing PD-1 and PD-L1 and enhancement of IFN-gamma production via PD-1/PD-L1 blockade in bovine mycoplasmosis. Immun Inflamm Dis 5:355–363. doi: 10.1002/iid3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikebuchi R, Konnai S, Okagawa T, Yokoyama K, Nakajima C, Suzuki Y, Murata S, Ohashi K. 2013. Blockade of bovine PD-1 increases T cell function and inhibits bovine leukemia virus expression in B cells in vitro. Vet Res 44:59. doi: 10.1186/1297-9716-44-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okagawa T, Konnai S, Nishimori A, Ikebuchi R, Mizorogi S, Nagata R, Kawaji S, Tanaka S, Kagawa Y, Murata S, Mori Y, Ohashi K. 2015. Bovine immunoinhibitory receptors contribute to suppression of Mycobacterium avium subsp. paratuberculosis-specific T-cell responses. Infect Immun 84:77–89. doi: 10.1128/IAI.01014-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okagawa T, Konnai S, Nishimori A, Maekawa N, Ikebuchi R, Goto S, Nakajima C, Kohara J, Ogasawara S, Kato Y, Suzuki Y, Murata S, Ohashi K. 2017. Anti-bovine programmed death-1 rat-bovine chimeric antibody for immunotherapy of bovine leukemia virus infection in cattle. Front Immunol 8:650. doi: 10.3389/fimmu.2017.00650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Merwe J, Prysliak T, Perez-Casal J. 2010. Invasion of bovine peripheral blood mononuclear cells and erythrocytes by Mycoplasma bovis. Infect Immun 78:4570–4578. doi: 10.1128/IAI.00707-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mulongo M, Prysliak T, Scruten E, Napper S, Perez-Casal J. 2014. In vitro infection of bovine monocytes with Mycoplasma bovis delays apoptosis and suppresses production of gamma interferon and tumor necrosis factor alpha but not interleukin-10. Infect Immun 82:62–71. doi: 10.1128/IAI.00961-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okagawa T, Konnai S, Deringer JR, Ueti MW, Scoles GA, Murata S, Ohashi K, Brown WC. 2016. Cooperation of PD-1 and LAG-3 contributes to T-cell exhaustion in Anaplasma marginale-infected cattle. Infect Immun 84:2779–2790. doi: 10.1128/IAI.00278-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hafalla JC, Claser C, Couper KN, Grau GE, Renia L, de Souza JB, Riley EM. 2012. The CTLA-4 and PD-1/PD-L1 inhibitory pathways independently regulate host resistance to Plasmodium-induced acute immune pathology. PLoS Pathog 8:e1002504. doi: 10.1371/journal.ppat.1002504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prysliak T, Van der Merwe J, Lawman Z, Wilson D, Townsend H, van Drunen Littel-van den Hurk S, Perez-Casal J. 2011. Respiratory disease caused by Mycoplasma bovis is enhanced by exposure to bovine herpes virus 1 (BHV-1) and not to bovine viral diarrhoea virus (BVDV) type 2. Can Vet J 52:1195–1202. [PMC free article] [PubMed] [Google Scholar]
- 33.Nicholas RA, Ayling RD. 2003. Mycoplasma bovis: disease, diagnosis, and control. Res Vet Sci 74:105–112. doi: 10.1016/S0034-5288(02)00155-8. [DOI] [PubMed] [Google Scholar]
- 34.Maunsell FP, Woolums AR, Francoz D, Rosenbusch RF, Step DL, Wilson DJ, Janzen ED. 2011. Mycoplasma bovis infections in cattle. J Vet Int Med 25:772–783. doi: 10.1111/j.1939-1676.2011.0750.x. [DOI] [PubMed] [Google Scholar]
- 35.Burki S, Spergser J, Bodmer M, Pilo P. 2016. A dominant lineage of Mycoplasma bovis is associated with an increased number of severe mastitis cases in cattle. Vet Microbiol 196:63–66. doi: 10.1016/j.vetmic.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 36.Tashiro H, Brenner MK. 2017. Immunotherapy against cancer-related viruses. Cell Res 27:59–73. doi: 10.1038/cr.2016.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Channappanavar R, Twardy BS, Suvas S. 2012. Blocking of PDL-1 interaction enhances primary and secondary CD8 T cell response to herpes simplex virus-1 infection. PLoS One 7:e39757. doi: 10.1371/journal.pone.0039757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vanden Bush TJ, Rosenbusch RF. 2004. Characterization of a lympho-inhibitory peptide produced by Mycoplasma bovis. Biochem Biophys Res Commun 315:336–341. doi: 10.1016/j.bbrc.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 39.Thomas CB, Mettler J, Sharp P, Jensen-Kostenbader J, Schultz RD. 1990. Mycoplasma bovis suppression of bovine lymphocyte response to phytohemagglutinin. Vet Immunol Immunopathol 26:143–155. doi: 10.1016/0165-2427(90)90063-X. [DOI] [PubMed] [Google Scholar]
- 40.Naot Y, Tully JG, Ginsburg H. 1977. Lymphocyte activation by various Mycoplasma strains and species. Infect Immun 18:310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song Z, Li Y, Liu Y, Xin J, Zou X, Sun W. 2012. α-Enolase, an adhesion-related factor of Mycoplasma bovis. PLoS One 7:e38836. doi: 10.1371/journal.pone.0038836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greenwald RJ, Freeman GJ, Sharpe AH. 2005. The B7 family revisited. Annu Rev Immunol 23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 43.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. 2007. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity 27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sachdev D, Gough KC, Flynn RJ. 2017. The chronic stages of bovine Fasciola hepatica are dominated by CD4 T-cell exhaustion. Front Immunol 8:1002. doi: 10.3389/fimmu.2017.01002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gagea MI, Bateman KG, Shanahan RA, van Dreumel T, McEwen BJ, Carman S, Archambault M, Caswell JL. 2006. Naturally occurring Mycoplasma bovis-associated pneumonia and polyarthritis in feedlot beef calves. J Vet Diagn Invest 18:29–40. doi: 10.1177/104063870601800105. [DOI] [PubMed] [Google Scholar]
- 46.Fulton RW, Blood KS, Panciera RJ, Payton ME, Ridpath JF, Confer AW, Saliki JT, Burge LT, Welsh RD, Johnson BJ, Reck A. 2009. Lung pathology and infectious agents in fatal feedlot pneumonias and relationship with mortality, disease onset, and treatments. J Vet Diagn Invest 21:464–477. doi: 10.1177/104063870902100407. [DOI] [PubMed] [Google Scholar]
- 47.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. 1999. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 11:141–151. doi: 10.1016/S1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 48.Kinter AL, Godbout EJ, McNally JP, Sereti I, Roby GA, O'Shea MA, Fauci AS. 2008. The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J Immunol 181:6738–6746. doi: 10.4049/jimmunol.181.10.6738. [DOI] [PubMed] [Google Scholar]
- 49.Perez-Casal J, Prysliak T. 2007. Detection of antibodies against the Mycoplasma bovis glyceraldehyde-3-phosphate dehydrogenase protein in beef cattle. Microb Pathog 43:189–197. doi: 10.1016/j.micpath.2007.05.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.