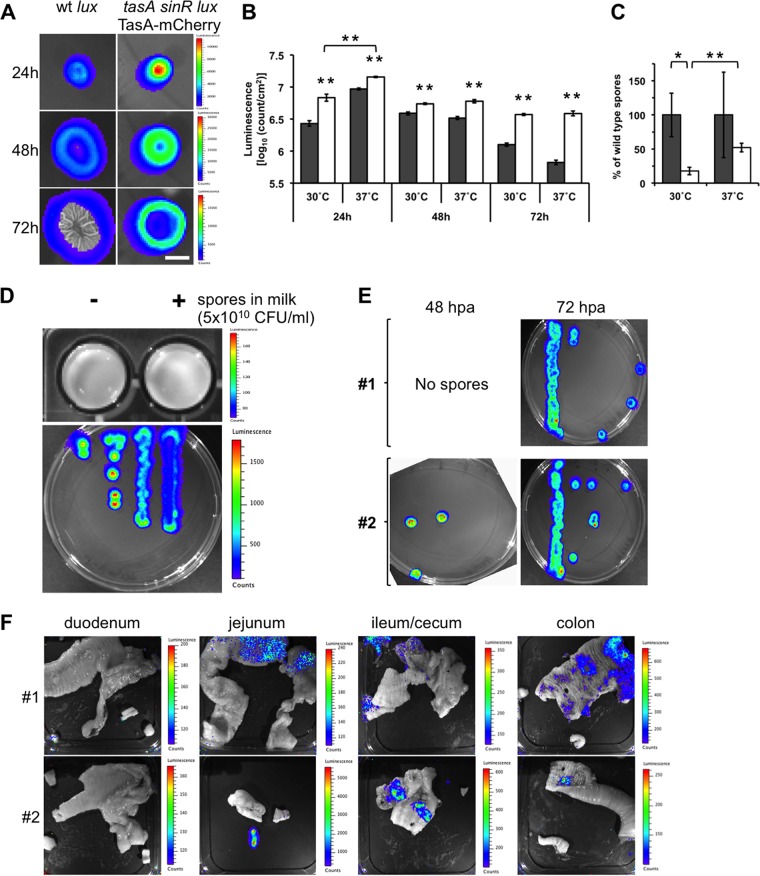
FIG 2.
Tracking of luminescent Bacillus subtilis in the guts of dogs. (A) Top view of luminescent biofilms from wt strain B. subtilis lux and B. subtilis tasA sinR lux TasA-mCherry incubated in MSgg semisolid medium for 24, 48, and 72 h at 30°C. A color luminescence scale is shown for each row. Bar, 1 cm. (B) Comparison of the luminescence of wt strain B. subtilis lux (gray bars) and B. subtilis tasA sinR lux TasA-mCherry (white bars) biofilms at 30°C and 37°C when incubated for 24, 48, and 72 h. The data represent the mean ± SEM (**, P < 0.01, t test, n = 4). (C) Sporulation ability of wt strain B. subtilis lux and B. subtilis tasA sinR lux TasA-mCherry from 72-h-old biofilms when incubated at 30°C and 37°C. The data represent the mean ± SEM (*, P < 0.05; **, P < 0.01; P values were determined by t test [n = 4]). (D) Comparison of the luminescence of spores resuspended in milk (top) and vegetative cells (bottom) from the B. subtilis tasA sinR lux TasA-mCherry strain. A color luminescence scale is shown for each panel. (E) Germination of spores isolated from anal swab samples from dogs 1 and 2 after the first oral application of B. subtilis tasA sinR lux TasA-mCherry spores. The anal swab samples were collected at 48 and 72 hpa. The spores were incubated in selective LB semisolid medium, and vegetative cells were monitored for luminescence. (F) Detection of luminescence from B. subtilis tasA sinR lux TasA-mCherry vegetative cells in the guts of dogs 1 and 2 after oral application of three doses of recombinant B. subtilis spores (5 × 1010 CFU on days 1, 21, and 42). Each picture corresponds to open portions of representative sections of the duodenum, jejunum, ileum/cecum, and colon of each animal. A color luminescence scale is shown for each picture. All luminescent images were acquired with a Xenogen IVIS camera and analyzed using Living Image (v4.0) software (Caliper Life Sciences, USA).