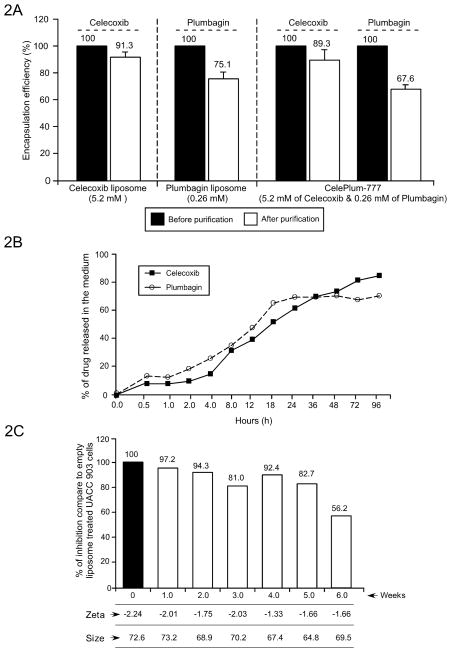
Figure 2. Characterization of drug encapsulation, stability, and release kinetics of CelePlum-777.
(2A). Drug encapsulation efficiency. Nanoliposomes containing Celecoxib alone (5.2 mM), Plumbagin alone (0.26 mM), or the combination (5.2 mM Celecoxib and 0.26 mM Plumbagin), Plumbagin encapsulation alone was 75.1%, Celecoxib encapsulation alone was 91.3% and the encapsulation of both agents was 67.6% and 89.3%, respectively. Data represent averages of at least 3 independent experiments; bars, S.E.M. (2B). Drug release kinetics of CelePlum-777. 71% of Celecoxib and 69% of Plumbagin was released from CelePlum-777 over 96 hours. (2C). Stability of CelePlum-777. CelePlum-777 was stored at 4°C and stability measured over weekly assessing size, charge, and cancer cell killing efficacy indicating stability for up to 5-weeks. Data represent averages of at least 2 independent experiments.