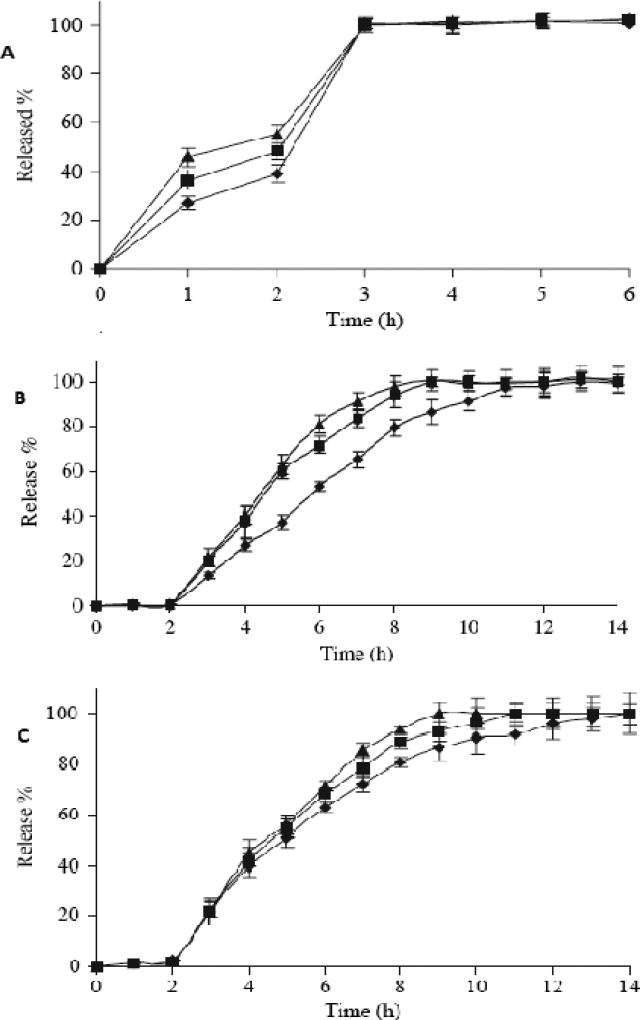
Figure 4.
Dissolution profiles of ketoprofen-Eudragit® L100 physical mixture tablets (A), ketoprofen-Eudragit® L100 tablets from extrudates (B), and ketoprofen-Eudragit® L100 tablets using pulverized extrudates (C) in 0.1 M HCl for the first 2 hours, then in pH 6.8 Phosphate buffer for subsequent hours (M ± SD, n = 6).
Formulation 1(▲: drug/polymer ratio 1:1); Formulation 2 (
 drug/polymer ratio 1:1.5); Formulation 3(◆: drug/polymer ratio 1:2).
drug/polymer ratio 1:1.5); Formulation 3(◆: drug/polymer ratio 1:2).