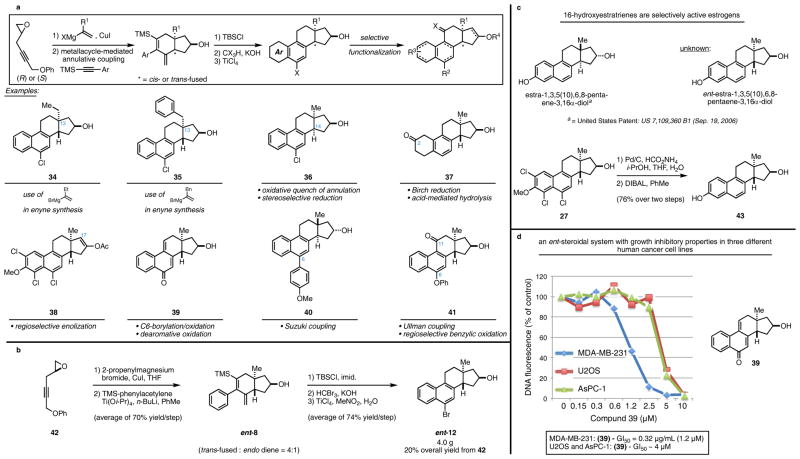
Figure 5.
a. Access to steroids with varying substitution and stereochemistry (see Supporting Information for experimental details associated with the syntheses of 34–41). b. Multigram-scale preparation of a synthetic ent-steroid. c. Preparation of ent-estra-1,3,5(10),6,8-pentaene-3,16α-diol. d. Discovery of an ent-steroid with cytotoxic properties: Cells were plated at 1000 cells/well of a 96 well plate. The following day, compound 39 was added in 2-fold dilutions (8 wells/concentration). After 7 days growth, cells were lyzed and analyzed for total DNA content as previously described52.